Abstract
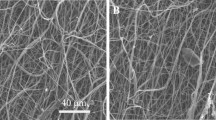
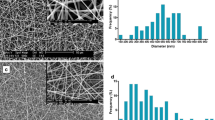
Urethral strictures were common disease caused by over-expression of extracellular matrix from fibroblast. In this study, we compare two nanoyarn scaffolds for improving fibroblasts infiltration without inhibition the over-expression of extracellular matrix. Collagen/poly(L-lactide-co-caprolactone) (Col/P(LLA-CL)) nanoyarn scaffolds were prepared by conjugated electrospinning and dynamic liquid electrospinning, respectively. In addition, co-axial electrospinning technique was combined with the nanoyarn fabrication process to produce nanoyarn scaffolds loading Wnt signaling pathway inhibitor. The mechanical properties of the scaffolds were examined and morphology was observed by SEM. Cell morphology, proliferation and infiltration on the scaffolds were investigated by SEM, MTT assay and H&E staining, respectively. The release profiles of different scaffolds were determined using HPLC. The results indicated that cells showed an organized morphology along the nanoyarns and considerable infiltration into the nanoyarn scaffolds prepared by dynamic liquid electrospinning (DLY). It was also observed that the DLY significantly facilitate cell proliferation. The D-DLY could facilitate the infiltration of the fibroblasts and could be a promising scaffold for the treatment of urethra stricture while it may inhibit the collagen production.
Similar content being viewed by others
References
van der Veer W M, Bloemen M C, Ulrich M M, et al. Potential cellular and molecular causes of hypertrophic scar formation. Burns, 2009, 35(1): 15–29
Zhang H, Ran X, Hu C L, et al. Therapeutic effects of liposomeenveloped Ligusticum chuanxiong essential oil on hypertrophic scars in the rabbit ear model. PLoS One, 2012, 7(2): e31157
Zhang K, Guo X, Zhao W, et al. Application of Wnt pathway inhibitor delivering scaffold for inhibiting fibrosis in urethra strictures: in vitro and in vivo study. International Journal of Molecular Sciences, 2015, 16(11): 27659–27676
Qi L, Song W, Liu Z, et al. Wnt3a promotes the vasculogenic mimicry formation of colon cancer via Wnt/ß-catenin signaling. International Journal of Molecular Sciences, 2015, 16(8): 18564–18579
Tan J, Tong B-D, Wu Y-J, et al. MicroRNA-29 mediates TGFß1- induced extracellular matrix synthesis by targeting wnt/ß-catenin pathway in human orbital fibroblasts. International Journal of Clinical and Experimental Pathology, 2014, 7(11): 7571–7577
Baarsma H A, Spanjer A I, Haitsma G, et al. Activation of WNT/ ß-catenin signaling in pulmonary fibroblasts by TGF-ß1 is increased in chronic obstructive pulmonary disease. PLoS One, 2011, 6(9): e25450
Bergmann C, Akhmetshina A, Dees C, et al. Inhibition of glycogen synthase kinase 3ß induces dermal fibrosis by activation of the canonical Wnt pathway. Annals of the Rheumatic Diseases, 2011, 70(12): 2191–2198
Conidi A, van den Berghe V, Huylebroeck D. Aptamers and their potential to selectively target aspects of EGF, Wnt/ß-catenin and TGFß-smad family signaling. International Journal of Molecular Sciences, 2013, 14(4): 6690–6719
Park K, Lee K, Zhang B, et al. Identification of a novel inhibitor of the canonical Wnt pathway. Molecular and Cellular Biology, 2011, 31(14): 3038–3051
Beyer C, Reichert H, Akan H, et al. Blockade of canonical Wnt signalling ameliorates experimental dermal fibrosis. Annals of the Rheumatic Diseases, 2013, 72(7): 1255–1258
Chuang P Y, Menon M C, He J C. Molecular targets for treatment of kidney fibrosis. Journal of Molecular Medicine, 2013, 91(5): 549–559
Hao S, He W, Li Y, et al. Targeted inhibition of ß-catenin/CBP signaling ameliorates renal interstitial fibrosis. Journal of the American Society of Nephrology, 2011, 22(9): 1642–1653
Langer R, Vacanti J P. Tissue engineering. Science, 1993, 260(5110): 920–926
Zhang Y, Venugopal J R, El-Turki A, et al. Electrospun biomimetic nanocomposite nanofibers of hydroxyapatite/chitosan for bone tissue engineering. Biomaterials, 2008, 29(32): 4314–4322
Wu J, Liu S, He L, et al. Electrospun nanoyarn scaffold and its application in tissue engineering. Materials Letters, 2012, 89: 146–149
Xu Y, Wu J, Wang H, et al. Fabrication of electrospun poly(Llactide-co-e-caprolactone)/collagen nanoyarn network as a novel, three-dimensional, macroporous, aligned scaffold for tendon tissue engineering. Tissue Engineering Part C: Methods, 2013, 19(12): 925–936
Di Lullo G A, Sweeney S M, Korkko J, et al. Mapping the ligandbinding sites and disease-associated mutations on the most abundant protein in the human, type I collagen. The Journal of Biological Chemistry, 2002, 277(6): 4223–4231
Kim B S, Mooney D J. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends in Biotechnology, 1998, 16(5): 224–230
Baker B M, Handorf A M, Ionescu L C, et al. New directions in nanofibrous scaffolds for soft tissue engineering and regeneration. Expert Review of Medical Devices, 2009, 6(5): 515–532
Huang C, Chen R, Ke Q, et al. Electrospun collagen-chitosan-TPU nanofibrous scaffolds for tissue engineered tubular grafts. Colloids and Surfaces B: Biointerfaces, 2011, 82(2): 307–315
Grover C N, Cameron R E, Best S M. Investigating the morphological, mechanical and degradation properties of scaffolds comprising collagen, gelatin and elastin for use in soft tissue engineering. Journal of the Mechanical Behavior of Biomedical Materials, 2012, 10: 62–74
Ji W, Sun Y, Yang F, et al. Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications. Pharmaceutical Research, 2011, 28(6): 1259–1272
Mirdailami O, Soleimani M, Dinarvand R, et al. Controlled release of rhEGF and rhbFGF from electrospun scaffolds for skin regeneration. Journal of Biomedical Materials Research Part A, 2015, 103(10): 3374–3385
Baker B M, Handorf A M, Ionescu L C, et al. New directions in nanofibrous scaffolds for soft tissue engineering and regeneration. Expert Review of Medical Devices, 2009, 6(5): 515–532
Lee H J, Lee S J, Uthaman S, et al. Biomedical applications of magnetically functionalized organic/inorganic hybrid nanofibers. International Journal of Molecular Sciences, 2015, 16(6): 13661–13677
Li X Y, Li Y C, Yu D G, et al. Fast disintegrating quercetin-loaded drug delivery systems fabricated using coaxial electrospinning. International Journal of Molecular Sciences, 2013, 14(11): 21647–21659
Stout D A. Recent advancements in carbon nanofiber and carbon nanotube applications in drug delivery and tissue engineering. Current Pharmaceutical Design, 2015, 21(15): 2037–2044
Bonkat G, Braissant O, Rieken M, et al. Comparison of the rollplate and sonication techniques in the diagnosis of microbial ureteral stent colonisation: results of the first prospective randomised study. World Journal of Urology, 2013, 31(3): 579–584
Lv Y. Nanofiber-based drug design, delivery and application. Current Pharmaceutical Design, 2015, 21(15): 1918–1919
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Guo, X., Zhang, K., El-Aassar, M. et al. The comparison of the Wnt signaling pathway inhibitor delivered electrospun nanoyarn fabricated with two methods for the application of urethroplasty. Front. Mater. Sci. 10, 346–357 (2016). https://doi.org/10.1007/s11706-016-0359-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11706-016-0359-3