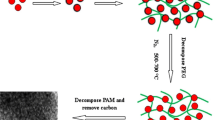
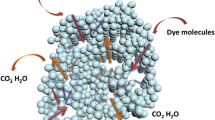
Abstract
This work demonstrated that mesoporous TiO2 (meso-TiO2) with controllable mesoporous and crystalline structures can be facilely prepared by using poly (ethylene glycol) (PEG) as structure-directing (SD) agent and peroxotitanic acid (PTA) as precursor. Meso-TiO2 with high specific surface area (157 m2∙g-1), pore volume (0.45 cm3∙g-1) and large mesopore size of 13.9 nm can be obtained after calcination at 450°C. Such meso-TiO2 also shows relatively high thermal stability. BET surface area still reaches 114 m2∙g-1 after calcination at 550°C. In the synthesis and calcination process, PEG that plays multiple and important roles in delivering thermally stable and tunable mesoporous and crystalline structures shows to be a suitable low-cost SD agent for the controllable preparation of nanocrystalline meso-TiO2. The photocatalytic activity tests show that both high surface area and bi-crystallinity of obtained meso-TiO2 are important in enhancing the performance in photo-decomposing Rhodamine B in water.
Similar content being viewed by others
References
Li W, Wu Z, Wang J, et al. A perspective on mesoporous TiO2 materials. Chemistry of Materials, 2014, 26(1): 287–298
Pan J H, Dou H, Xiong Z, et al. Porous photocatalysts for advanced water purifications. Journal of Materials Chemistry, 2010, 20(22): 4512–4528
Antonelli D M, Ying J Y. Synthesis of hexagonally packed mesoporous TiO2 by a modified sol–gel method. Angewandte Chemie International Edition, 1995, 34(18): 2014–2017
Zi S C, Chandren S, Yuan L S, et al. New method to synthesize mesoporous titania by photodegradation of surfactant template. Solid State Sciences, 2016, 52: 83–91
Tong H, Enomoto N, Inada M, et al. Synthesis of mesoporous TiO2 spheres and aggregates by sol–gel method for dye-sensitized solar cells. Materials Letters, 2015, 141: 259–262
Oveisi H, Suzuki N, Beitollahi A, et al. Aerosol-assisted fabrication of mesoporous titania spheres with crystallized anatase structures and investigation of their photocatalitic properties. Journal of Sol-Gel Science and Technology, 2010, 56(2): 212–218
Shamaila S, Sajjad A K L, Chen F, et al. Mesoporous titania with high crystallinity during synthesis by dual template system as an efficient photocatalyst. Catalysis Today, 2011, 175(1): 568–575
Samiee L, Beitollahi A, Vinu A. Effect of calcination atmosphere on the structure and photocatalytic properties of titania mesoporous powder. Research on Chemical Intermediates, 2012, 38(7): 1467–1482
Shibata H, Ogura T, Mukai T, et al. Direct synthesis of mesoporous titania particles having a crystalline wall. Journal of the American Chemical Society, 2005, 127(47): 16396–16397
Tian C X, Yang Y, Pu H. Effect of calcination temperature on porous titania prepared from industrial titanyl sulfate solution. Applied Surface Science, 2011, 257(20): 8391–8395
Chu S, Luo L L, Yang J C, et al. Low-temperature synthesis of mesoporous TiO2 photocatalyst with self-cleaning strategy to remove organic templates. Applied Surface Science, 2012, 258(24): 9664–9667
Masolo E, Senes N, Pellicer E, et al. Evaluation of the anatase/ rutile phase composition influence on the photocatalytic performances of mesoporous TiO2 powders. International Journal of Hydrogen Energy, 2015, 40(42): 14483–14491
Crepaldi E L, Soler-Illia G J, Grosso D, et al. Controlled formation of highly organized mesoporous titania thin films: from mesostructured hybrids to mesoporous nanoanatase TiO2. Journal of the American Chemical Society, 2003, 125(32): 9770–9786
Choi S Y, Mamak M, Coombs N, et al. Thermally stable twodimensional hexagonal mesoporous nanocrystalline anatase, meso-nc-TiO2: Bulk and crack-free thin film morphologies. Advanced Functional Materials, 2004, 14(4): 335–344
Zhou W, Sun F, Pan K, et al. Well-ordered large-pore mesoporous anatase TiO2 with remarkably high thermal stability and improved crystallinity: preparation, characterization, and photocatalytic performance. Advanced Functional Materials, 2011, 21(10): 1922–1930
Smarsly B, Grosso D, Brezesinski T, et al. Highly crystalline cubic mesoporous TiO2 with 10-nm pore diameter made with a new block copolymer template. Chemistry of Materials, 2004, 16(15): 2948–2952
Zhang J, Deng Y, Gu D, et al. Ligand-assisted assembly approach to synthesize large-pore ordered mesoporous titania with thermally stable and crystalline framework. Advanced Energy Materials, 2011, 1(2): 241–248
Ahn S H, Chi W S, Kim D J, et al. Honeycomb-like organized TiO2 photoanodes with dual pores for solid-state dye-sensitized solar cells. Advanced Functional Materials, 2013, 23(31): 3901–3908
Sallard S, Schröder M, Boissière C, et al. Bimodal mesoporous titanium dioxide anatase films templated by a block polymer and an ionic liquid: influence of the porosity on the permeability. Nanoscale, 2013, 5(24): 12316–12329
Wang W, Nguyen D, Long H, et al. High temperature and waterbased evaporation induced self-assembly approach for facile and rapid synthesis of nanocrystalline mesoporous TiO2. Journal of Materials Chemistry A: Materials for Energy and Sustainability, 2014, 2(38): 15912–15920
Nguyen D, Wang W, Long H, et al. Synthesis,characterization and photoactivity of bi-crystalline mesoporous TiO2. Frontiers of Materials Science, 2016, 10(1): 23–30
Nguyen D, Wang W, Long H, et al. A facile and controllable multi-templating approach based on a solo nonionic surfactant to preparing nanocrystalline bimodal meso-mesoporous titania. Microporous and Mesoporous Materials, 2016, 230: 177–187
Zhang H Z, Banfield J F. Understanding Polymorphic phase transformation behavior during growth of nanocrystalline aggregates: insights from TiO2. Journal of Materials Chemistry B: Materials for Biology and Medicine, 2000, 104(15): 3481–3487
Zhang D Y, Yang D, Zhang H J, et al. Synthesis and photocatalytic properties of hollow microparticles of titania and titania/carbon composites templated by Sephadex G-100. Chemistry of Materials, 2006, 18(15): 3477–3485
Wanka G, Hoffmann H, Ulbricht W. Phase diagrams and aggregation behavior of poly(oxyethy1ene)-poly(oxypropylene)- poly(oxyethylene) triblock copolymers in aqueous solutions. Macromolecules, 1994, 27(15): 4145–4159
Yusuf M M, Imai H, Hirashima H. Preparation of mesoporous titania by templating with polymer and surfactant and its characterization. Journal of Sol-Gel Science and Technology, 2003, 28(1): 97–104
Sun X, Zheng C, Qiao M, et al. Bioinspired synthesis of hierarchical macro-mesoporous titania with tunable macroporous morphology using cell-assemblies as macrotemplates. Chemical Communications, 2009, 31(31): 4750–4752
Tu L, Pan H, Xie H X, et al. Study on the fabrication and photovoltaic property of TiO2 mesoporous microspheres. Solid State Sciences, 2012, 14(5): 616–621
Lee J, Orilall M C, Warren S C, et al. Direct access to thermally stable and highly crystalline mesoporous transition-metal oxides with uniform pores. Nature Materials, 2008, 7(3): 222–228
Zhang R, Tu B, Zhao D. Synthesis of highly stable and crystalline mesoporous anatase by using a simple surfactant sulfuric acid carbonization method. Chemistry, 2010, 16(33): 9977–9981
Yamada S, Wang Z, Mouri E, et al. Crystallization of titania ultrafine particles from peroxotitanic acid in aqueous solution in the present of polymer and incorporation into poly (methyl methacylate) via dispersion in organic solvent. Colloid & Polymer Science, 2009, 287(2): 139–146
Bacsa R R, Kiwi J. Effect of rutile phase on the photocatalytic properties of nanocrystalline titania during the degradation of pcoumaric acid. Applied Catalysis B: Environmental, 1998, 16(1): 19–29
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nguyen, D., Wang, W., Long, H. et al. Facile and controllable preparation of mesoporous TiO2 using poly(ethylene glycol) as structure-directing agent and peroxotitanic acid as precursor. Front. Mater. Sci. 10, 405–412 (2016). https://doi.org/10.1007/s11706-016-0352-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11706-016-0352-x