Abstract
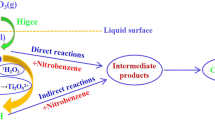
This study investigated the indirect oxidation of nitrobenzene (NB) by hydroxyl radicals (·OH) in a rotating packed bed (RPB) using competitive kinetics method with p-nitrochlorobenzene as a reference compound. The rate constants of NB with ·OH are calculated to be between (1.465±0.113) × 109 L/(mol·s) and (2.497±0.192) × 109 L/(mol·s). The experimental data are fitted by the modified Arrhenius equation, where the activation energy is 4877.74 J/mol, the order of NB concentration, rotation speed, and initial pH is 0.2425, 0.1400 and 0.0167, respectively. The ozonation process of NB could be enhanced by RPB, which is especially effective for highly concentrated NB-containing waste-water under alkaline conditions. The high gravity technology can accelerate ozone mass transfer and self-decomposition of ozone to produce more ·OH, resulting in an increase in the indirect oxidation rate of NB by ·OH and consequently effective degradation of NB in wastewater.

Similar content being viewed by others
References
Liu H T, Sui M H, Yuan B J, Wang J Y, Lv Y N. Efficient degradation of nitrobenzene by Cu-Co-Fe-LDH catalyzed peroxymonosulfate to produce hydroxyl radicals. Chemical Engineering Journal, 2019, 357: 140–149
Palmisano G, Loddo V, Augugliaro V, Palmisano L, Yurdakal S. Photocatalytic oxidation of nitrobenzene and phenylamine: pathways and kinetics. AIChE Journal. American Institute of Chemical Engineers, 2010, 53: 961–968
Jiao W Z, Yang P Z, Gao W Q, Qiao J J, Liu Y Z. Apparent kinetics of the ozone oxidation of nitrobenzene in aqueous solution enhanced by high gravity technology. Chemical Engineering and Processing-Process Intensification, 2019, 146: 107690
Jiao W Z, Qin Y J, Luo S, He Z, Feng Z R, Liu Y Z. Simultaneous formation of nanoscale zero-valent iron and degradation of nitrobenzene in wastewater in an impinging stream-rotating packed bed reactor. Chemical Engineering Journal, 2017, 321: 564–571
Duan H T, Liu Y, Yin X H, Bai J F, Qi J. Degradation of nitrobenzene by Fenton-like reaction in a H2O2/schwertmannite system. Chemical Engineering Journal, 2016, 283: 873–879
Yang P Z, Luo S, Liu Y Z, Jiao W Z. Degradation of nitrobenzene wastewater in an acidic environment by Ti(IV)/H2O2/O3 in a rotating packed bed. Environmental Science and Pollution Research International, 2018, 25: 25060–25070
Elshafei G M S, Yehia F Z, Dimitry O H, Badawi A M, Eshaq G. Ultrasonic assisted-Fenton-like degradation of nitrobenzene at neutral pH using nanosized oxides of Fe and Cu. Ultrasonics Sonochemistry, 2014, 21: 1358–1365
Wu J, Su T M, Jiang Y X, Xie X L, Qin Z Z, Ji H B. Catalytic ozonation of cinnamaldehyde to benzaldehyde over CaO: experiments and intrinsic kinetics. AIChE Journal. American Institute of Chemical Engineers, 2017, 63: 4403–4417
Martins R C, Cardoso M, Dantas R F, Sans C, Esplugas S, QuintaFerreira R M. Catalytic studies for the abatement of emerging contaminants by ozonation. Journal of Chemical Technology and Biotechnology (Oxford, Oxfordshire), 2015, 90: 1611–1618
Huber M M, Canonica S, Park G Y, Von Gunten U. Oxidation of pharmaceuticals during ozonation and advanced oxidation processes. Environmental Science & Technology, 2013, 37: 1016–1024
Sun X M, Wu C Y, Zhou Y X, Han W. Using DOM fraction method to investigate the mechanism of catalytic ozonation for real wastewater. Chemical Engineering Journal, 2019, 369: 100–108
Chiang Y P, Liang Y Y, Chang C N, Chao A C. Differentiating ozone direct and indirect reactions on decomposition of humic substances. Chemosphere, 2006, 65: 2395–2400
Beltrán F J, Encinar J M, Alonso M A. Nitroaromatic hydrocarbon ozonation in water. 1. Single ozonation. Industrial & Engineering Chemistry Research, 1998, 37: 25–31
Hoigné J, Bader H. Rate constants of reactions of ozone with organic and inorganic compounds in water—I: non-dissociating organic compounds. Water Research, 1983, 17(2): 173–183
Jiao W Z, Luo S, He Z, Liu Y Z. Applications of high gravity technologies for wastewater treatment: a review. Chemical Engineering Journal, 2017, 313: 912–927
Burns J R, Ramshaw C. Process intensification: visual study of liquid maldistribution in rotating packed beds. Chemical Engineering Science, 1996, 51: 1347–1352
Chen Y H, Chang C Y, Su W L, Chiu C Y, Yu Y H, Chiang P C, Chang C F, Shie J L, Chiou C S. Chiang S I M. Ozonation of CI Reactive Black 5 using rotating packed bed and stirred tank reactor. Journal of Chemical Technology and Biotechnology, 2015, 80: 68–75
Chiang C Y, Chen Y S, Liang M S, Lin F Y, Tai C Y D, Liu H S. Absorption of ethanol into water and glycerol/water solution in a rotating packed bed. Journal of the Taiwan Institute of Chemical Engineers, 2009, 40: 418–423
Luo Y, Chu G W, Zhou H K, Zhao Z Q, Dudukovic M P, Chen J F. Gas-liquid effective interfacial area in a rotating packed bed. Industrial & Engineering Chemistry Research, 2012, 51: 16320–16352
Zeng Z Q, Zhou H K, Li X, Arowo M, Sun B C, Chen J F, Chu G W, Shao L. Degradation of phenol by ozone in the presence of Fenton reagent in a rotating packed bed. Chemical Engineering Journal, 2013, 229: 404–411
Jiao W Z, Liu Y Z, Liu W L, Li J, Shao F, Wang C R. Degradation of nitrobenzene-containing wastewater with O3 and H2O2 by high gravity technology. China Petroleum Processing and Petrochemical Technology, 2013, 15(1): 85–94
Guo L, Jiao W Z, Liu Y Z, Xu C C, Liu W L, Li J. Treatment of nitrobenzene-containing wastewater using different combined processes with ozone. Chinese Journal Energetic Materials, 2014, 22(5): 702–708
Yang P Z, Luo S, Liu H Y, Jiao W Z, Liu Y Z. Aqueous ozone decomposition kinetics in a rotating packed bed. Journal of the Taiwan Institute of Chemical Engineers, 2019, 96: 11–17
Jung Y, Hong E, Kwon M, Kang J W. A kinetic study of ozone decay and bromine formation in saltwater ozonation: effect of O3 dose, salinity, pH, and temperature. Chemical Engineering Journal, 2017, 312: 30–38
Chu W, Ma C W. Quantitative prediction of direct and indirect dye ozonation kinetics. Water Research, 2000, 34: 3153–3160
Zhao Y, Yu G, Chen S Y, Zhang S Y, Wang B, Huang J, Deng S B, Wang Y J. Ozonation of antidepressant fluoxetine and its metabolite product norfluoxetine: kinetics, intermediates and toxicity. Chemical Engineering Journal, 2017, 316: 951–963
Chen W R, Wu C, Elovitz M S, Linden K G, Suffet I H. Reactions of thiocarbamate, triazine and urea herbicides, RDX and benzenes on EPA Contaminant Candidate List with ozone and with hydroxyl radicals. Water Research, 2008, 42(1–2): 137–144
Hoigné J. Inter-calibration of ·OH radical sources and water quality parameters. Water Science and Technology, 1997, 35: 1–8
Leitner N K V, Roshani B. Kinetic of benzotriazole oxidation by ozone and hydroxyl radical. Water Research, 2010, 44(6): 2058–2066
El Najjar N H, Touffet A, Deborde M, Journel R, Leitner N K V. Levofloxacin oxidation by ozone and hydroxyl radicals: kinetic study, transformation products and toxicity. Chemosphere, 2013, 93(4): 604–611
Wang Z Y, Shao Y S, Gao N Y, An A. Degradation kinetic of dibutyl phthalate (DBP) by sulfate radical-and hydroxyl radical-based advanced oxidation process in UV/persulfate system. Separation and Purification Technology, 2018, 195: 92–100
Shen J M, Chen Z L, Xu Z Z, Li X Y, Xu B B, Qi F. Kinetics and mechanism of degradation of p-chloronitrobenzene in water by ozonation. Journal of Hazardous Materials, 2008, 152: 1325–1331
Li B Z, Xu X, Zhu L. Ozonation ofchloronitrobenzenes in aqueous solution: kinetics and mechanism. Journal of Chemical Technology and Biotechnology, 2009, 84: 167–175
Wang F, Wang Y, Ji M. Mechanisms and kinetics models for ultrasonic waste activated sludge disintegration. Journal of Hazardous Materials, 2005, 123: 145–150
Ko C H, Guan C Y, Lu P J, Chern J M. Ozonation of guaiacol solution in a rotating packed bed. Chemical Engineering Journal, 2011, 171: 1045–1052
Zeng Z Q, Zou H K, Li X, Sun B C, Chen J F, Shao L. Ozonation of phenol with O3/Fe(II) in acidic environment in a rotating packed bed. Industrial & Engineering Chemistry Research, 2012, 51: 10509–10516
Zhao W R, Wu Z B, Wang D H. Ozone direct oxidation kinetics of cationic red X-GRL in aqueous solution. Journal of Hazardous Materials, 2006, 137(3): 1859–1865
Zhao W R, Liu F F, Yang Y, Tan M, Zhao D Y. Ozonation of cationic red X-GRL in aqueous solution: kinetics and modeling. Journal of Hazardous Materials, 2004, 57: 1189–1199
Matthews R W, Sangster D F. Measurement by benzoate radiolytic decarboxylation of relative rate constants for hydroxyl radical reactions. Journal of Physical Chemistry, 1965, 69(6): 1938–1946
Chen Y H, Chang C Y, Su W L, Chen C C, Chiu C Y, Yu Y H, Chiang P C, Chiang S I M. Modeling ozone contacting process in a rotating packed bed. Industrial & Engineering Chemistry Research, 2004, 43(1): 228–236
Hoigné J, Bader H. The role of hydroxyl radical reactions in ozonation processes in aqueous solutions. Water Research, 1976, 10(5): 377–386
Acknowledgements
This work was supported by the Specialized Research Fund for Sanjin Scholars Program of Shanxi Province (No. 201707), Key Research & Development Plan of Shanxi Province (No. 201903D321059), Shanxi Scholarship Council of China (No. HGKY2019071), and Transformation and Cultivation Projects of Scientific and Technological Achievements of Higher Education Institutions for Shanxi Province (No. 2020CG040).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiao, W., Shao, S., Yang, P. et al. Kinetics and mechanism of nitrobenzene degradation by hydroxyl radicals-based ozonation process enhanced by high gravity technology. Front. Chem. Sci. Eng. 15, 1197–1205 (2021). https://doi.org/10.1007/s11705-020-1998-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-020-1998-6