Abstract
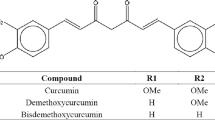
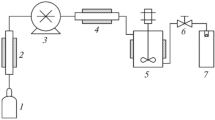
Curcumin is a hydrophobic polyphenol compound exhibiting a wide range of biological activities such as anti-inflammatory, anti-bacterial, anti-fungal, anti-carcinogenic, anti-human immunodeficiency virus, and antimicrobial activity. In this work, a swirl mixer was employed to produce the micronized curcumin with polyvinylpyrrolidone (PVP) by the supercritical anti-solvent process to improve the bioavailability of curcumin. The effects of operating parameters such as curcumin/PVP ratio, feed concentration, temperature, pressure, and CO2 flow rate were investigated. The characterization and solubility of particles were determined by using scanning electron microscopy, Fourier Transform Infrared spectroscopy, and ultra-violet-visible spectroscopy. The result shows that the optimal condition for the production of curcumin/PVP particles is at curcumin/PVP ratio of 1:30, feed concentration of 5 mg·mL−1, temperature of 40 °C, pressure of 15 MPa, and CO2 flow rate of 15 mL·min−1. Moreover, the dissolution of curcumin/PVP particles is faster than that of raw curcumin.

Similar content being viewed by others
References
Moghadamtousi S Z, Kadir H A, Hassandarvish P, Tajik H, Abubakar S, Zandi K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Research Internaitonal, 2014, 2014: 1–12
Anand P, Kunnumakkara A B, Newman R A, Aggarwal B B. Bioavailability of curcumin: Problems and promise. Molecular Pharmaceutics, 2007, 4(6): 807–818
Montes A, Gordillo M D, Pereyra C, Martínez de la Ossa E J. Polymer and ampicillin co-precipitation by supercritical antisolvent process. Journal of Supercritical Fluids, 2012, 63: 92–98
Fernández-Ponce MT, Masmoudi Y, Djerafi R, Casas L, Mantell C, Monrtínez de la Ossa E, Badens E. Particle design applied to quercetin using supercritical anti-solvent techniques. Journal of Supercritical Fluids, 2015, 105: 119–127
Adami R, Capua A D, Reverchon E. Supercritical assisted atomization for the production of curcumin-biopolymer microspheres. Powder Technology, 2017, 305: 455–461
Zabihi F, Xin N, Jia J, Chen T, Zhao Y. High yield and high loading preparation of curcumin-PLGA nanoparticles using a modified supercritical antisolvent technique. Industrial & Engineering Chemistry Research, 2014, 53(15): 6569–6574
Ha E S, Choo G H, Beak I H, Kim M S. Formulation, characterization, and in vivo evaluation of celecoxib-PVP solid dispersion nanoparticles using supercritical anti-solvent coprecipitation. Molecules (Basel, Switzerland), 2014, 19(12): 20325–20339
Zahran F, Cabañas A, Cheda J A R, Renuncio J A R, Pando C. Dissolution rate enhancement of anti-inflammatory drug diflunisal by coprecipitation with a biocompaticle polymer using carbon dioxide as a supercritical fluid antisolvent. Journal of Supercritical Fluids, 2014, 88: 56–65
Prosapio V, De Macro I, Scognamiglio M, Reverchon E. Folic acid-PVP nanostructured composite microparticles by supercritical antisolvent precipitation. Chemical Engineering Journal, 2015, 277: 286–294
Kurniawansyah F, Mammucari R, Foster N R. Inhalable curcumin formulations by supercritical technology. Powder Technology, 2015, 284: 289–298
Prosapio V, De Marco I, Reverchon E. PVP/corticosteroid microspheres produced by supercritical antisolvent coprecipitation. Chemical Engineering Journal, 2016, 292: 264–275
Montes A, Wehner L, Pereyra C, Martínez De La Ossa E J. Generation of microparticles of ellagic acid by supercritical antisolvent process. Journal of Supercritical Fluids, 2016, 116: 101–110
Prosapio V, Reverchon E, De Marco I. Formulation of PVP/nimesulide microspheres by supercritical antisolvent coprecipitation. Journal of Supercritical Fluids, 2016, 118: 19–26
Montes A, Wehner L, Pereyra C, De La Ossa E J M. Mangiferin nanoparticles precipitation by supercritical antisolvent process. Journal of Supercritical Fluids, 2016, 112: 44–50
Xie M, Li Y, Zao Z, Chen A, Li J, Hu J, Li G, Li Z. Solubility enhancement of curcumin via supercritical CO2 based silk fibroin carrier. Journal of Supercritical Fluids, 2015, 103: 1–9
Jia J, Song N, Gai Y, Zhang L, Zhao Y. Release-controlled curcumin proliposome produced by ultrasound-assisted supercritical antisolvent method. Journal of Supercritical Fluids, 2016, 113: 150–157
Pedro A S, Villa S D, Caliceti P, De Melo S A B V, Albuquerque E C, Bertucco A, Salmaso S. Curcumin-loaded solid lipid particles by PGSS technology. Journal of Supercritical Fluids, 2016, 107: 534–541
Baldino L, Cardea S, Reverchon E. Biodegradable membranes loaded with curcumin to be used as engineered independent devices in active packaging. Journal of the Taiwan Institute of Chemical Engineers, 2017, 71: 518–526
Kawasaki S, Sue K, Ookawara R, Wakashima Y, Suzuki A. Development of novel micro swirl mixer for producing fine metal oxide nanoparticles by continuous supercritical hydrothermal method. Journal of Oleo Science, 2010, 59(10): 557–562
Patomchaiviwat V, Paeratakul O, Kulvanich P. Formation of inhalable rifampicin-poly(L-lactide) microparticles by supercritical anti-solvent process. America Association of Pharmaceutical Scientists, 2008, 9(4): 1119–1129
Reverchon E, De Marcro I, Della Porta G. Tailoring of nano-and micro-particle of some superconductor precursors by supercritical antisolvent precipitation. Journal of Supercritical Fluids, 2002, 23 (1): 81–87
De Marco I, Reverchon E. Influence of pressure, temperature, and concentration on the mechanisms of particle precipitation in supercritical antisolvent micronization. Journal of Supercritical Fluids, 2011, 58(2): 295–302
Anwar M, Ahmad I, Warsi M H, Mohapatra S, Ahmad N, Akhter S, Ali A, Almad F J. Experimental investigation and oral bioavailability enhancement of nano-sized curcumin by using supercritical anti-solvent process. European Journal of Pharmaceutics and Biopharmaceutics, 2015, 96: 162–172
Li Y, Yu Y, Wang H, Zhao F. Effect of process parameters on the recrystallization and the size control of puerarin using the supercritical fluid antisolvent process. Asian Journal of Pharmaceutical Sciences, 2016, 11(2): 281–291
Li W, Liu G, Li L, Wu J, Lü Y, Jiang Y. The effect of process parameters on co-precipitation of paclitaxel and Poly(L-lactic acid) by supercritical antisolvent. Chinese Journal of Chemical Engineering, 2012, 20(4): 803–813
Miguel F, Martín A, Gamse T, Cocero M J. Supercritical anti solvent precipitation of lycopene: Effect of the operating parameters. Journal of Supercritical Fluids, 2006, 36(3): 225–235
Su C, Lo W, Lien L. Micronization of fluticasone propionate using supercritical antisolvent process. Chemical Engineering & Technology, 2011, 34(4): 535–541
Careno S, Boutin O, Badens E. Drug recrystallization using supercritical anti-solvent (SAS) process with impinging jets: Effect of process parameters. Journal of Crystal Growth, 2012, 342(1): 34–41
Kim M, Lee S, Park J, Woo J, Hwang S. Micronization of cilostazol using supercritical antisolvent (SAS) process: Effect of process parameters. Powder Technology, 2007, 177(2): 64–70
Reverchon E. Supercritical antisolvent precipitation of micro-and nano-particles. Journal of Supercritical Fluids, 1999, 15(1): 1–21
Martín A, Mattea F, Gutiérrez K, Miguel F, Cocero M J. Coprecipitation of carotenoids and bio-polymers with supercritical anti-solvent process. Journal of Supercritical Fluids, 2007, 41(1): 138–147
Yen F, Wu T, Tzeng C W, Lin L, Lin C. Curcumin nanoparticle improve the physicochemical properties of curcumin and effectively enhance its antioxidant and antithepatoma activities. Journal of Agriculture and Food Chemistry, 2010, 58(12): 73–76-7382
Uzun I N, Sipahigil O, Dinçer S. Coprecipitation of cefuroxime axetil-PVP composite microparticles by batch supercritical antisolvent process. Journal of Supercritical Fluids, 2011, 55(3): 1059–1069
Perrut M, Jung J, Leboeuf F. Enhancement of dissolution rate of poorly-soluble active ingredients by supercritical fluid processes: Part 1: Micronization of neat particles. International Journal of Pharmaceutics, 2005, 288(1): 3–10
Acknowledgements
This research is supported by ASEAN University Network for Southeast Asia Engineering Education Development Network (AUN/SEED-Net) project through the Japan International Cooperation Agency (JICA) and the Precursory Research for Embryonic Science and Technology Program of the Japan Science and Technology Agency (JST).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chhouk, K., Wahyudiono, Kanda, H. et al. Micronization of curcumin with biodegradable polymer by supercritical anti-solvent using micro swirl mixer. Front. Chem. Sci. Eng. 12, 184–193 (2018). https://doi.org/10.1007/s11705-017-1678-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-017-1678-3