Abstract
Rhamnolipids are one of the most effective biosurfactants that are of great interest in industrial applications such as enhancing oil recovery, health care, cosmetics, pharmaceutical processes, food processing, detergents for protein folding, and bioremediation due to their unique characteristics such as low toxicity, surface active property to reduce surface/interfacial tensions, and excellent biodegradability. The genes and metabolic pathways for rhamnolipid synthesis have been well elucidated, but its cost-effective production is still challenging. Pseudomonas aeruginosa, the most powerful rhamnolipid producer, is an opportunistic pathogen, which limits its large scale production and applications. Rhamnolipid production using engineered strains other than Pseudomonas aeruginosa such as E. coli and Pseudomonas putida has received much attention. The highest yield of rhamnolipids is achieved when oil-type carbon sources are used, but using cheaper and renewable carbon sources such as lignocellulose would be an attractive strategy to reduce the production cost of rhamnolipids for various industrial applications.

Similar content being viewed by others
References
Henkel M, Müller M M, Kügler J H, Lovaglio R B, Contiero J, Syldatk C, Hausmann R. Rhamnolipids as biosurfactants from renewable resources: Concepts for next-generation rhamnolipid production. Process Biochemistry, 2012, 47(8): 1207–1219
Shekhar S, Sundaramanickam A, Balasubramanian T. Biosurfactant producing microbes and their potential applications: A review. Critical Reviews in Environmental Science and Technology, 2015, 45(14): 1522–1554
Desai J D, Banat I M. Microbial production of surfactants and their commercial potential. Microbiology and Molecular Biology Reviews, 1997, 61(1): 47–64
Banat I M, Franzetti A, Gandolfi I, Bestetti G, Martinotti M G, Fracchia L, Smyth T J, Marchant R. Microbial biosurfactants production, applications and future potential. Applied Microbiology and Biotechnology, 2010, 87(2): 427–444
Banat I M, Marchant A, Nigam P, Gaston S J, Kelly B A, Marchant R. Production, partial characterization, and potential diagnostic use of salicylate hydroxylase from Pseudomonas putida UUC-1. Enzyme and Microbial Technology, 1994, 16(8): 665–670
Deziel E, Lepine F, Dennie D, Boismenu D, Mamer O A, Villemur R. Liquid chromatography/mass spectrometry analysis of mixtures of rhamnolipids produced by Pseudomonas aeruginosa strain 57RP grown on mannitol or naphthalene. Biochimica et Biophysica Acta, 1999, 1440(2-3): 244–252
Abdel-Mawgoud A M, Lepine F, Deziel E. Rhamnolipids: Diversity of structures, microbial origins and roles. Applied Microbiology and Biotechnology, 2010, 86(5): 1323–1336
Cha M, Lee N, Kim M, Lee S. Heterologous production of Pseudomonas aeruginosa EMS1 biosurfactant in Pseudomonas putida. Bioresource Technology, 2008, 99(7): 2192–2199
Gunther N, Nunez A, Fett W, Solaiman D K. Production of rhamnolipids by Pseudomonas chlororaphis, a nonpathogenic bacterium. Applied and Environmental Microbiology, 2005, 71(5): 2288–2293
Janek T, Lukaszewicz M, Krasowska A. Identification and characterization of biosurfactants produced by the Arctic bacterium Pseudomonas putida BD2. Colloids and Surfaces. B, Biointerfaces, 2013, 110: 379–386
Rooney A P, Price N P, Ray K J, Kuo T M. Isolation and characterization of rhamnolipid-producing bacterial strains from a biodiesel facility. FEMS Microbiology Letters, 2009, 295(1): 82–87
Lovaglio R B, Silva V L, Ferreira H, Hausmann R, Contiero J. Rhamnolipids know-how: Looking for strategies for its industrial dissemination. Biotechnology Advances, 2015, 33(8): 1715–1726
Pantazaki A A, Papaneophytou C P, Lambropoulou D A. Simultaneous polyhydroxyalkanoates and rhamnolipids production by Thermus thermophilus HB8. AMB Express, 2011, 1(1): 17
Abouseoud M, Maachi R, Amrane A, Boudergua S, Nabi A. Evaluation of different carbon and nitrogen sources in production of biosurfactant by Pseudomonas fluorescens. Desalination, 2008, 223(1-3): 143–151
Lang S, Wullbrandt D. Rhamnose lipids—biosynthesis, microbial production and application potential. Applied Microbiology and Biotechnology, 1999, 51(1): 22–32
Banat I M, Makkar R S, Cameotra S S. Potential commercial applications of microbial surfactants. Applied Microbiology and Biotechnology, 2000, 53(5): 495–508
Lovaglio R B, dos Santos F J, Jafelicci M Jr, Contiero J. Rhamnolipid emulsifying activity and emulsion stability: pH rules. Colloids and Surfaces. B, Biointerfaces, 2011, 85(2): 301–305
Li Q, Kang C, Wang H, Liu C, Zhang C. Application of microbial enhanced oil recovery technique to Daqing Oilfield. Biochemical Engineering Journal, 2002, 11(2-3): 197–199
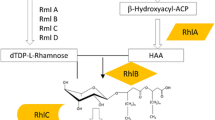
Rahim R, Burrows L L, Monteiro M A, Perry M B, Lam J S. Involvement of the rml locus in core oligosaccharide and O polysaccharide assembly in Pseudomonas aeruginosa. Microbiology, 2000, 146: 2803–2814
Olvera C, Goldberg J B, Sanchez R, Soberon-Chavez G. The Pseudomonas aeruginosa algC gene product participates in rhamnolipid biosynthesis. FEMS Microbiology Letters, 1999, 179(1): 85–90
Aguirre-Ramirez M, Medina G, Gonzalez-Valdez A, Grosso-Becerra V, Soberon-Chavez G. The Pseudomonas aeruginosa rmlBDAC operon, encoding dTDP-l-rhamnose biosynthetic enzymes, is regulated by the quorum-sensing transcriptional regulator RhlR and the alternative sigma factor sigmaS. Microbiology, 2012, 158: 908–916
Marumo K, Lindqvist L, Verma N, Weintraub A, Reeves P R, Lindberg A A. Enzymatic synthesis and isolation of thymidine diphosphate-6-deoxy-d-xylo-4-hexulose and thymidine diphosphate-l-rhamnose. Production using cloned gene products and separation by HPLC. European Journal of Biochemistry, 1992, 204(2): 539–545
Ochsner U A, Fiechter A, Reiser J. Isolation, characterization, and expression in Escherichia coli of the Pseudomonas aeruginosa rhlAB genes encoding a rhamnosyltransferase involved in rhamnolipid biosurfactant synthesis. Journal of Biological Chemistry, 1994, 269(31): 19787–19795
Kutchma A J, Hoang T T, Schweizer H P. Characterization of a Pseudomonas aeruginosa fatty acid biosynthetic gene cluster: purification of acyl carrier protein (ACP) and malonyl-coenzyme A: ACP transacylase (FabD). Journal of Bacteriology, 1999, 181(17): 5498–5504
Hoang T T, Schweizer H P. Characterization of Pseudomonas aeruginosa enoyl-acyl carrier protein reductase (FabI): A target for the antimicrobial triclosan and its role in acylated homoserine lactone synthesis. Journal of Bacteriology, 1999, 181(17): 5489–5497
Hoang T T, Schweizer H P. Fatty acid biosynthesis in Pseudomonas aeruginosa: Cloning and characterization of the fabAB operon encoding beta-hydroxyacyl-acyl carrier protein dehydratase (FabA) and beta-ketoacyl-acyl carrier protein synthase I (FabB). Journal of Bacteriology, 1997, 179(17): 5326–5332
Ochsner U A, Reiser J. Autoinducer-mediated regulation of rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. Proceedings of the National Academy of Sciences of the United States of America, 1995, 92(14): 6424–6428
Ochsner U A, Koch A K, Fiechter A, Reiser J. Isolation and characterization of a regulatory gene affecting rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. Journal of Bacteriology, 1994, 176(7): 2044–2054
Parsek M R, Val D L, Hanzelka B L, Cronan J E Jr, Greenberg E P. Acyl homoserine-lactone quorum-sensing signal generation. Proceedings of the National Academy of Sciences of the United States of America, 1999, 96(8): 4360–4365
Medina G, Juarez K, Soberon-Chavez G. The Pseudomonas aeruginosa rhlAB operon is not expressed during the logarithmic phase of growth even in the presence of its activator RhlR and the autoinducer N-butyryl-homoserine lactone. Journal of Bacteriology, 2003, 185(1): 377–380
Fuqua C, Greenberg E P. Self perception in bacteria: Quorum sensing with acylated homoserine lactones. Current Opinion in Microbiology, 1998, 1(2): 183–189
Dobler L, Vilela L F, Almeida R V, Neves B C. Rhamnolipids in perspective: Gene regulatory pathways, metabolic engineering, production and technological forecasting. New Biotechnology, 2016, 33(1): 123–135
Dusane D H, Zinjarde S S, Venugopalan V P, McLean R J, WeberM M, Rahman P K. Quorum sensing: Implications on rhamnolipid biosurfactant production. Biotechnology & Genetic Engineering Reviews, 2010, 27: 159–184
Benincasa M, Contiero J, Manresa M A, Moraes I O. Rhamnolipid production by Pseudomonas aeruginosa LBI growing on soapstock as the sole carbon source. Journal of Food Engineering, 2002, 54(4): 283–288
Muller M M, Hormann B, Syldatk C, Hausmann R. Pseudomonas aeruginosa PAO1 as a model for rhamnolipid production in bioreactor systems. Applied Microbiology and Biotechnology, 2010, 87(1): 167–174
Sim L, Ward O P, Li Z Y. Production and characterisation of a biosurfactant isolated from Pseudomonas aeruginosa UW-1. Journal of Industrial Microbiology & Biotechnology, 1997, 19(4): 232–238
Reiling H E, Thanei-Wyss U, Guerra-Santos L H, Hirt R, Kappeli O, Fiechter A. Pilot plant production of rhamnolipid biosurfactant by Pseudomonas aeruginosa. Applied and Environmental Microbiology, 1986, 51(5): 985–989
Wittgens A, Tiso T, Arndt T T, Wenk P, Hemmerich J, Muller C, Wichmann R, Kupper B, Zwick M, Wilhelm S, Hausmann R, Syldatk C, Rosenau F, Blank L M. Growth independent rhamnolipid production from glucose using the non-pathogenic Pseudomonas putida KT2440. Microbial Cell Factories, 2011, 10: 80
Banat I M, Satpute S K, Cameotra S S, Patil R, Nyayanit N V. Cost effective technologies and renewable substrates for biosurfactants’ production. Frontiers in Microbiology, 2014, 5: 1–18
Makkar R, Cameotra S. An update on the use of unconventional substrates for biosurfactant production and their new applications. Applied Microbiology and Biotechnology, 2002, 58(4): 428–434
Wei Y H, Chou C L, Chang J S. Rhamnolipid production by indigenous Pseudomonas aeruginosa J4 originating from petrochemical wastewater. Biochemical Engineering Journal, 2005, 27(2): 146–154
Müller M M, Kügler J H, Henkel M, Gerlitzki M, Hörmann B, Pöhnlein M, Syldatk C, Hausmann R. Rhamnolipids—Next generation surfactants? Journal of Biotechnology, 2012, 162(4): 366–380
Wu J Y, Yeh K L, Lu W B, Lin C L, Chang J S. Rhamnolipid production with indigenous Pseudomonas aeruginosa EM1 isolated from oil-contaminated site. Bioresource Technology, 2008, 99(5): 1157–1164
Shreve G S, Inguva S, Gunnam S. Rhamnolipid biosurfactant enhancement of hexadecane biodegradation by Pseudomonas aeruginosa. Molecular Marine Biology and Biotechnology, 1995, 4(4): 331–337
Arino S, Marchal R, Vandecasteele J P. Identification and production of a rhamnolipidic biosurfactant by a Pseudomonas species. Applied Microbiology and Biotechnology, 1996, 45(1): 162–168
Trummler K, Effenberger F, Syldatk C. An integrated microbial/ enzymatic process for production of rhamnolipids and l-(+)-rhamnose from rapeseed oil with Pseudomonas sp. DSM 2874. European Journal of Lipid Science and Technology, 2003, 105(10): 563–571
Chen S Y, Lu W B, Wei Y H, Chen W M, Chang J S. Improved production of biosurfactant with newly isolated Pseudomonas aeruginosa S2. Biotechnology Progress, 2007, 23(3): 661–666
Jeong H S, Lim D J, Hwang S H, Ha S D, Kong J Y. Rhamnolipid production by Pseudomonas aeruginosa immobilised in polyvinyl alcohol beads. Biotechnology Letters, 2004, 26(1): 35–39
de Sousa J R, da Costa Correia J A, de Almeida J G L, Rodrigues S, Pessoa O D L, Melo V M M, Gonçalves L R B. Evaluation of a coproduct of biodiesel production as carbon source in the production of biosurfactant by P. aeruginosa MSIC02. Process Biochemistry, 2011, 46(9): 1831–1839
Nitschke M, Costa S G, Haddad R, Goncalves L A, Eberlin M N, Contiero J. Oil wastes as unconventional substrates for rhamnolipid biosurfactant production by Pseudomonas aeruginosa LBI. Biotechnology Progress, 2005, 21(5): 1562–1566
Benincasa M, Abalos A, Oliveira I, Manresa A. Chemical structure, surface properties and biological activities of the biosurfactant produced by Pseudomonas aeruginosa LBI from soapstock. Antonie van Leeuwenhoek, 2004, 85(1): 1–8
Nitschke M, Costa S G, Contiero J. Structure and applications of a rhamnolipid surfactant produced in soybean oil waste. Applied Biochemistry and Biotechnology, 2010, 160(7): 2066–2074
Benincasa M, Accorsini F R. Pseudomonas aeruginosa LBI production as an integrated process using the wastes from sunflower-oil refining as a substrate. Bioresource Technology, 2008, 99(9): 3843–3849
de Lima C J, Franca F P, Servulo E F, Resende M M, Cardoso V L. Enhancement of rhamnoplipid production in residual soybean oil by an isolated strain of Pseudomonas aeruginosa. Applied Biochemistry and Biotechnology, 2007, 137–140(1): 463–470
Abalos A, Pinazo A, Infante M R, Casals M, García F, Manresa A. Physicochemical and antimicrobial properties of new rhamnolipids produced by Pseudomonas aeruginosa AT10 from soybean oil refinery wastes. Langmuir, 2001, 17(5): 1367–1371
Raza Z A, Khan M S, Khalid Z M, Rehman A. Production kinetics and tensioactive characteristics of biosurfactant from a Pseudomonas aeruginosa mutant grown on waste frying oils. Biotechnology Letters, 2006, 28(20): 1623–1631
Haba E, Espuny M J, Busquets M, Manresa A. Screening and production of rhamnolipids by Pseudomonas aeruginosa 47T2 NCIB 40044 from waste frying oils. Journal of Applied Microbiology, 2000, 88(3): 379–387
Mercadé M E, Manresa M A, Robert M, Espuny M J, de Andrés C, Guinea J. Olive oil mill effluent (OOME). New substrate for biosurfactant production. Bioresource Technology, 1993, 43(1): 1–6
Kaskatepe B, Yildiz S, Gumustas M, Ozkan S A. Biosurfactant production by Pseudomonas aeruginosain kefir and fish meal. Brazilian Journal of Microbiology, 2015, 46(3): 855–859
Sudhakar B P, Vaidya A N, Bal A S, Kapur R, Juwarkar A, Khanna P. Kinetics of biosurfactant production by Pseudomonas aeruginosa strain BS2 from industrial wastes. Biotechnology Letters, 1996, 18(3): 263–268
Dubey K, Juwarkar A. Distillery and curd whey wastes as viable alternative sources for biosurfactant production. World Journal of Microbiology & Biotechnology, 2001, 17(1): 61–69
Koch A K, Reiser J, Kappeli O, Fiechter A. Genetic construction of lactose-utilizing strains of Pseudomonas aeruginosa and their application in biosurfactant production. Nature Biotechnology, 1988, 6(11): 1335–1339
Colak A K, Kahraman H. The use of raw cheese whey and olive oil mill wastewater for rhamnolipid production by recombinant Pseudomonas aeruginosa. Environmental and Experimental Biology, 2013, 11: 125–135
Raza Z A, Ahmad N, Kamal S. Multi-response optimization of rhamnolipid production using grey rational analysis in Taguchi method. Biotechnology Reports (Amsterdam, Netherlands), 2014, 3: 86–94
Patel R M, Desai A J. Biosurfactant production by Pseudomonas aeruginosa GS3 from molasses. Letters in Applied Microbiology, 1997, 25(2): 91–94
Gudiña E J, Rodrigues A I, Alves E, Domingues M R, Teixeira J A, Rodrigues L R. Bioconversion of agro-industrial by-products in rhamnolipids toward applications in enhanced oil recovery and bioremediation. Bioresource Technology, 2015, 177: 87–93
Raza Z A, Khan M S, Khalid Z M. Physicochemical and surfaceactive properties of biosurfactant produced using molasses by a Pseudomonas aeruginosa mutant. Journal of Environmental Science and Health. Part A, Toxic/Hazardous Substances & Environmental Engineering, 2007, 42(1): 73–80
Prabu R, Kuila A, Ravishankar R, Rao P V C, Choudary N V, Velankar H R. Microbial rhamnolipid production in wheat straw hydrolysate supplemented with basic salts. RSC Advances, 2015, 5(64): 51642–51649
Henkel M, Schmidberger A, Vogelbacher M, Kuhnert C, Beuker J, Bernard T, Schwartz T, Syldatk C, Hausmann R. Kinetic modeling of rhamnolipid production by Pseudomonas aeruginosa PAO1 including cell density-dependent regulation. Applied Microbiology and Biotechnology, 2014, 98(16): 7013–7025
Syldatk C, Lang S, Matulovic U, Wagner F. Production of four interfacial active rhamnolipids from n-alkanes or glycerol by resting cells of Pseudomonas species DSM 2874. Zeitschrift für Naturforschung. C, 1985, 40(1-2): 61–67
Dumont M J, Narine S S. Characterization of soapstock and deodorizer distillates of vegetable oils using gas chromatography. Lipid Technology, 2008, 20(6): 136–138
Makkar R S, Cameotra S S, Banat I M. Advances in utilization of renewable substrates for biosurfactant production. AMB Express, 2011, 1(1): 5
Keegstra K. Plant cell walls. Plant Physiology, 2010, 154(2): 483–486
Morais S, Morag E, Barak Y, Goldman D, Hadar Y, Lamed R, Shoham Y, Wilson D B, Bayer E A. Deconstruction of lignocellulose into soluble sugars by native and designer cellulosomes. mBio, 2012, 3(6): 214104
Li Q, Ng WT, Wu J C. Isolation, characterization and application of a cellulose-degrading strain Neurospora crassa S1 from oil palm empty fruit bunch. Microbial Cell Factories, 2014, 13(1): 157
Miller E N, Jarboe L R, Turner P C, Pharkya P, Yomano L P, York S W, Nunn D, Shanmugam K T, Ingram L O. Furfural inhibits growth by limiting sulfur assimilation in ethanologenic Escherichia coli strain LY180. Applied and Environmental Microbiology, 2009, 75(19): 6132–6141
Koopman F, Wierckx N, de Winde J H, Ruijssenaars H J. Identification and characterization of the furfural and 5-(hydroxymethyl) furfural degradation pathways of Cupriavidus basilensis HMF14. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(11): 4919–4924
Lawniczak L, Marecik R, Chrzanowski L. Contributions of biosurfactants to natural or induced bioremediation. Applied Microbiology and Biotechnology, 2013, 97(6): 2327–2339
Mukherjee S, Das P, Sen R. Towards commercial production of microbial surfactants. Trends in Biotechnology, 2006, 24(11): 509–515
Perfumo A, Rudden M, Smyth T J, Marchant R, Stevenson P S, Parry N J, Banat I M. Rhamnolipids are conserved biosurfactants molecules: Implications for their biotechnological potential. Applied Microbiology and Biotechnology, 2013, 97(16): 7297–7306
Soberon-Chavez G, Lepine F, Deziel E. Production of rhamnolipids by Pseudomonas aeruginosa. Applied Microbiology and Biotechnology, 2005, 68(6): 718–725
Aktiengesellschaft H. Pseudomonas aeruginosa and its use in a process for the biotechnological preparation of l-rhamnose. US Patents, 5501966 A, 1996
Ochsner U A, Reiser J, Fiechter A, Witholt B. Production of Pseudomonas aeruginosa rhamnolipid biosurfactants in heterologous hosts. Applied and Environmental Microbiology, 1995, 61(9): 3503–3506
Cabrera-Valladares N, Richardson A P, Olvera C, Trevino L G, Deziel E, Lepine F, Soberon-Chavez G. Monorhamnolipids and 3-(3-hydroxyalkanoyloxy)alkanoic acids (HAAs) production using Escherichia coli as a heterologous host. Applied Microbiology and Biotechnology, 2006, 73(1): 187–194
Guerra-Santos L, Kappeli O, Fiechter A. Pseudomonas aeruginosa biosurfactant production in continuous culture with glucose as carbon source. Applied and Environmental Microbiology, 1984, 48(2): 301–305
Gudina E J, Fernandes E C, Rodrigues A I, Teixeira J A, Rodrigues L R. Biosurfactant production by Bacillus subtilis using corn steep liquor as culture medium. Frontiers in Microbiology, 2015, 6: 59
Pauly M, Keegstra K. Plant cell wall polymers as precursors for biofuels. Current Opinion in Plant Biology, 2010, 13(3): 305–312
Zhang D, Ong Y L, Li Z, Wu J C. Optimization of dilute acidcatalyzed hydrolysis of oil palm empty fruit bunch for high yield production of xylose. Chemical Engineering Journal, 2012, 181–182: 636–642
Li Q, Ng W T, Puah S M, Bhaskar R V, Soh L S, Macbeath C, Parakattil P, Green P, Wu J C. Efficient production of fermentable sugars from oil palm empty fruit bunch by combined use of acid and whole cell culture-catalyzed hydrolyses. Biotechnology and Applied Biochemistry, 2014, 61(4): 426–431
Acknowledgements
This research is supported by the Science and Engineering Research Council (SERC) of the Agency for Science, Technology and Research (A*STAR) of Singapore (SERC grant number: 1526004161).
Author information
Authors and Affiliations
Corresponding author
Additional information
Dr. Qingxin Li is currently a scientist at ICES, A*STAR, Singapore. She received her Ph.D. in Microbiology from Shandong University. She received further postdoctoral trainings in Nanyang Technological University and Vanderbilt University. She was trained in microbiology, biochemistry, enzymology, structural biology, and biotechnology. Her current research interests are to convert wastes from food industries to value added chemicals that can be used in food production and drug discovery. She is working with both academia and industries to explore versatile applications of microbiology techniques to food industries. She has been working on converting Empty Fruit Bunch (EFB)-a common waste from palm oil production to fermentable sugars. She is also interested in production of biosurfactants using sugars produced from EFB, production of d-lactic acid with high purity, and folding of important enzymes in different biosurfactants. She has been using structural information to improve thermal stability of important enzymes.
Rights and permissions
About this article
Cite this article
Li, Q. Rhamnolipid synthesis and production with diverse resources. Front. Chem. Sci. Eng. 11, 27–36 (2017). https://doi.org/10.1007/s11705-016-1607-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-016-1607-x