Abstract
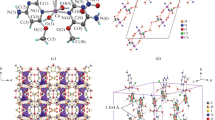
It is known, that in the Cu4OX6L4 (X = Cl, Br) complexes can be present many different ligands L, including bioligands. The synthesis and characterization of Cu4OBCl6(ron)4 (1) and Cu4OCl6(3-Mepy)4 (2) (where ron is ronicol or 3-methanolpyridine and 3-Mepy is 3-methylpyridine) are reported. The complexes under study were X-ray structure analysis and Hirshfeld surface analysis. Tetranuclear Cu4OX6L4 complexes with molecular structure (Fig. 1) can help to better understand the role of donor–acceptor and electron-transfer properties in copper proteins. The coordination sphere about each copper(II) atom is trigonal bipyramidal with three chlorine atoms in the equatorial plane. The apical positions are occupied by the central oxygen atom and the nitrogen atom of the respective ligand (CuCl3ON). Here are studied chloridocomplexes of some N-donor ligands, L = chloro-promazine, ronicol (3-pyridylmethanol), 2-ethylpyrazine, seven derivatives of pyrazol and for comparisons 3-methylpyridine. The Cu4OCl6L4 molecule is regarded as a supramolecular model of interactions between bioligand L and hypothetical “round-shaped” coordination tetra-receptor Cu4OCl6. Vector calculations applied usualy to mechanical and electrical macroconstructions are here applied to microconstructions represented by structures of Cu4OX6L4 molecules. For vector calculations each Cu4OX6L4 structure is placed (Fig. 1) into the three-dimensional Cartesian coordinate system with the central oxygen atom O1 placed in its origin 0. Studied bioligands are compared and described by molecular structural dynamics and corresponding shifts of electron densities by means of bond lenghts (O1–Cu, Cu–L, Cu–X) and structural distances (O1···X, O1···L).

Structure of the Cu4OX6L4 molecule








Similar content being viewed by others
References
Atria AM, Vega A, Contreras M, Valenzuela J, Spodine EC (1999) Magnetostructural characterization of μ4-oxahexa-μ2-chlorotetrakis(imidazole)copper(II). Inorg Chem 38:5681–5685. https://doi.org/10.1021/ic990389
Becker S, Behrens U, Schindler S (2015) Investigations concerning [Cu4OX6L4] cluster formation of copper(II) chloride with amine ligands related to benzylamine. Eur J Inorg Chem 2015:2437–2447. https://doi.org/10.1002/ejic.201500115
Betanzos-Lara S, Gomez-Ruiz C, Barron-Sosa LR, Gracia-Mora I, Flores-Alamo M, Barba-Behrens N (2012) Cytotoxic copper(II), cobalt(II), zinc(II), and nickel(II) coordination compounds of clotrimazole. J Inorg Biochem 114:82–93. https://doi.org/10.1016/j.jinorgbio.2012.05.001
Bowmaker GA, Nicola CD, Marchetti F, Pettinari C, Skelton BW, Somers N, White AH (2011) Synthesis, spectroscopic and structural characterization of some novel adducts of copper(II) salts with unidentate nitrogen bases. Inorg Chim Acta 375:31–40. https://doi.org/10.1016/j.ica.2011.04.005
Burla MC, Caliandro R, Camalli M, Carrozzini B, Cascarano GL, Giacovazzo C, Mallamo M, Mazzone A, Polidori G, Spagna R (2012) SIR2011: a new package for crystal structure determination and refinement. J Appl Crystallogr 45:357–361. https://doi.org/10.1107/S0021889812001124
Červeň I (2015) Fyzika po kapitolách 1 Vektory. Vydavateľstvo Fakulty elektrotechniky a informatiky STU, Bratislava
Cikunov E (1973) Zbierka matematických vzorcov. ALFA, vydavateľstvo technickej a ekonomickej literatúry, Bratislava
Cortes P, Atria AM, Garland MT, Baggio R (2006) Three oxo complexes with a tetranuclear [Cu4(μ2-Cl)6(μ4-O)] unit. Acta Crystallogr Sect C 62:m311–m314. https://doi.org/10.1107/S0108270106021354
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) OLEX2: a complete structure solution, refinement and analysis program. J Appl Crystallogr 42:339–341. https://doi.org/10.1107/S0021889808042726
El-Toukhy A, Cai GZ, Davies G, Gilbert TR, Onan KD, Veidis D (1984) Transmetalation reactions of tetranuclear copper(II) complexes. 2. Steichiometry and products of reactions of [(DENC)CuCl]4O2, [(DENC)CuCl]4(CO3)2, [(DENC)CuCl]4Cl4, and (DENC)4Cu4Cl6O complexes (DENC = N, N-diethylnicotinamide) with Ni(NS)2 complexes (nS is an S-methyl hydrazinecarbodithioate Schiff Base), the kinetics of product isomerization in aprotic solvents, and inhibition of copper-catalyzed phenolic oxidative coupling by dioxygen through transmetalation. J Am Chem Soc 106:4596–4605. https://doi.org/10.1021/ja00328a050
Gill N, Sterns M (1970) The preparation and properties of µ4-oxo-hexa-µ-chloro-tetrakis[(2-methylpyridine)copper(II)] hydrate, Cu4OCl6(2-mepy)4ˑxH2O, and di-µ-methoxo-bis[chloro)2-methylpyridine)copper(II)], [CuCl(OCH3)(2-mepy)]2, and X-ray structure analysis of Cu4OCl6(2-mepy)4ˑxH2O. Inorg Chem 9:1619–1625. https://doi.org/10.1021/ic50089a004
Groom CR, Bruno IJ, Lightfoot MP, Ward SC (2016) The cambridge structural database. Acta Crystallogr Sect B 72:171–179. https://doi.org/10.1107/S2052520616003954
He HS (2011) Hexa-μ2-chlorido-μ4-oxido-tetrakis[(3-methyl-5-phenyl-1H-pyrazole-κN2)copper(II)]. Acta Crystallogr Sect E E 67:m 140. https://doi.org/10.1107/S1600536810053663
Hirshfeld FL (1977) Bonded-atom fragments for describing molecular charge densities. Theor Chim Acta 44:129–138. https://doi.org/10.1007/BF00549096
Jacimovic ZK, Leovac VM, Tomic ZD (2007) Crystal structure of hexakis(μ2-chloro)-μ4-oxo-tetrakis((3,5-dimethyl-pyrazole)copper(II)) ethanol tetrasolvate, Cu4OCl6(C5H8N2)4∙4C2H5OH. Z Kristallogr New Cryst Struct 224:246–248. https://doi.org/10.1524/ncrs.2007.0103
Janiak C (2000) A critical account on π-π stacking in metal complexes with aromatic nitrogen-containing ligand. J Chem Soc Dalton Trans 2000:3885–3896. https://doi.org/10.1039/B0030100
Jian FF, Zhao PS, Wang HX, Lu LD (2004) Hydrothermal synthesis, crystal structure and EPR property of tetranuclear copper(II) cluster [Cu4OCl6(C14H12N2)4]. Bull Korean Chem Soc 25:673–675. https://doi.org/10.5012/bkcs.2004.25.5.673
Kariuki BM, Newman PD (2018) Asymmetric cationic phosphines: synthesis, coordination chemistry, and reactivity. Inorg Chem 57:9554–9563. https://doi.org/10.1021/acs.inorgchem.8b01657
Kashyap S, Singh UP, Singh AK, Kumar P, Singh SP (2013) Synthesis and structural studies of some copper-benzoate complexes. Transit Met Chem 38:573–585. https://doi.org/10.1007/s11243-013-9725-5
Keij FS, Haasnoot JG, Oosterling AJ, Reedijk J, Connor CJO, Zhang JH, Spek AL (1991) A pyrazole ligand yielding both chloro-bridged dinuclear and tetranuclear copper(II) compounds. The crystal and molecular structure of bis[μ-chloro-chloro(3,4-dimethyl-5-phenylpyrazole) (4,5-dimethyl-3-phenylpyrazole)copper(II)] and of (μ4-oxo)hexakis(μ-chloro)tetrakis(3,4-dimethyl-5-phenylpyrazole)tetracopper(II). Inorg Chim Acta 181:185–193. https://doi.org/10.1016/S0020-1693(00)86809-7
Koman M, Ondrejovič G (2013) Ligand ronicol, which brings together and divides. Adv Sci Eng Med 5:598–602. https://doi.org/10.1166/asem.2013.1327
Liu XM, Kilner CA, Halcrow MA (2003) Hexa-μ2-chloro-μ4-oxo-tetrakis{[5-(2,4,6-trimethylphenyl)pyrazole-κN2]copper(II)}. Acta Crystallogr Sect C 59:m100–m102. https://doi.org/10.1107/S0108270103002853
Lobana TS, Sultana R, Butcher RJ (2011) A sandmeyer type reaction for bromination of mercapto-1-methyl-imidazoline (N2C4H6S) into 2-bromo-1-methyl-imidazole (N2C4H5Br) in presence of copper(II) bromide. Dalton Trans 40:11382–11384. https://doi.org/10.1039/C1DT11327E
Löw S, Becker J, Würtele C, Miska A, Kleeberg C, Behrens U, Walter O, Schindler S (2013) Reactions of copper(II) chloride in solution: facile formation of tetranuclear copper clusters and other complexes that are relevant in catalytic redox processes. Chem Eur J 19:5342–5351. https://doi.org/10.1002/chem.201203848
Melník M, Koman M, Ondrejovič G (2011) Tetramers Cu4(μ4-O)(μ-X)6(L4): analysis of structural data. Coord Chem Rev 255:1581–1586. https://doi.org/10.1016/j.ccr2010.12.005
Nather C, Jess I (2002) Hexa-μ2-chloro-tetrakis(2-ethylpyrazine-N)-μ4-oxo-tetracopper(II). Acta Crystallogr Sect E 58:m4–m6. https://doi.org/10.1107/S1600536801020438
Norman RE, Rose NJ (1989) Simple, direct synthesis and structure of Hexa-μ-chloro-tetrakis- (1-methylimidazole)-μ4-oxo-tetracopper(II). Acta Crystallogr C 45:1707–1713. https://doi.org/10.1107/S0108270189002994
Ondrejovič G, Kotočová A (2006) Spectral and electrochemical study of coordination molecules Cu4OX6L4: 3-pyridylmethanol and 4-pyridylmethanol Cu4OBrnCl(6-n)(pm)4 complexes. Chem Pap 60:198–206. https://doi.org/10.2478/s11696-006-0036-6
Ondrejovič G, Moncoľ J (2015) Structures of Cu4OCl6L4 complexes studied by vector analysis. Book of Abstracts—XXV. International conference on coordination and bioinorganic chemistry, Smolenice: 101
Ondrejovič G, Moncoľ J (2017) Advanced structural analysis of coordination Cu4OX6L4 molecules. Book of Abstracts—XXVI. International conference on coordination and bioinorganic chemistry, smolenice: 82
Ondrejovič G, Broškovičová A, Kotočová A (2000) Construction and structure of coordination polymers based on tetragonal Cu4OBr 6 centres linked by pyrazine. Chem Pap 54:6–11
Parkin A, Barr G, Dong W, Gilmore CJ, Jayalitaka D, McKinnon JJ, Spackman MA, Wilson CC (2007) Comparing entire crystal structures: structural genetic fingerprinting. CrystEngComm 9:648–652. https://doi.org/10.1039/B704177B
Richardson C, Steel PJ (2003) Benzotriazole as a structural component in chelating and bridging heterocyclic ligands; ruthenium, palladium, copper and silver complexes. Dalton Trans 2003:992–1000. https://doi.org/10.1039/B206990C
Sheldrick GM (2015a) SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr A 71:3–8. https://doi.org/10.1107/S2053273314026370
Sheldrick GM (2015b) Crystal structure refinement with SHELXL. Acta Crystallogr C 71:3–8. https://doi.org/10.1107/S2053229614024218
Siemens XE (1990) Siemens analytical X–ray Instruments Inc. Madison, Wisconsin
Skorda K, Stamatatos TC, Vafiadis AP, Lithoxoidou AT, Terzis A, Perlepes SP, Mrozinski J, Raptopoulou CP, Plakatouras JC, Bakalbassis EG (2005) Copper(II) chloride/1-methylbenzotriazole chemistry: influence of various synthetic parameters on the product identity, structural and magnetic characterization, and quantum-chemical studies. Inorg Chim Acta 358:565–582. https://doi.org/10.1016/j.ica.2004.09.042
Spackman MA, Jayalitaka D (2009) Hirshfeld surface analysis. CrystEngComm 11:19–32. https://doi.org/10.1039/B818330A
Spackman MA, McKinnon JJ (2002) Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 4:378–392. https://doi.org/10.1039/B203191B
Stibrany RT, Potenza JA (2007) CCDC 666757: experimental crystal structure determination. https://doi.org/10.5517/ccqct95
Tosik A, Bukowska-Strzyzewska M, Mrozinski J (2009) Synthesis, magnetism and X-ray structure of μ4-oxo-hexa-μ2-chlorotetrakis(benzimidazole)copper(II). J Coord Chem 24:113–125. https://doi.org/10.1080/00958979109409454
Turner MJ, McKinnon JJ, Wolff SK, Gromwood DJ, Spackman PR, Jayalitaka D, Spackman MA (2017) CrystalExplorer17.5. The University of Western Australia, Crawley
Vafazadeh R, Willis AC (2016) Synthesis, structure and electrochemistry of tetranuclear oxygen-centered copper(II) clusters with acetylacetone and benz-pyrazole hydrolyzed derivatives as ligands. Acta Chim Slov 63:186–192. https://doi.org/10.17344/acsi.2016.2263
Vafazadeh R, Hasanzade N, Heidari MM, Willis AC (2015) Synthesis, structure characterization, DNA binding, and cleavage properties of mononuclear and tetranuclear cluster of copper(II) complexes. Acta Chim Slov 62:122–129. https://doi.org/10.17344/acsi.2014.797
van Albada GA, Ghazzali M, Al-Farhan K, Reedijk J (2011) Synthesis and crystal structure of (µ4-oxido)hexakis(µ-chlorido)tetrakis(2-(3-pyridyl)ethane-1-ol)tetracopper(II). A compound with a unique hydrogen bond system stabilizing the network. Inorg Chem Commun 14:1149–1152. https://doi.org/10.1016/j.inoche.2011.04.010
Voitekhovich SV, Gaponik PN, Lyakhov AS, Filipova JV, Sukhanova AG, Sukhanov GT, Ivashkevich OA (2009) N-Alkylation of 4-nitro-1,2,3-triazole revisited. Detection and characterization of the N3-ethylation product, 1-ethyl-5-nitro-1,2,3-triazole. Tetrahedron Lett 50:2577–2579. https://doi.org/10.1016/j.tetlet.2009.03.076
Yamada K, Oguma E, Nakagawa H, Kawazura H (1994) Structure of μ-Oxo-tetranuclear Copper(II) complex produced from chlorpromazine (cpz) and Cu(II)Cl2. An alternative binding site of the cpz molecule to metal. Chem Pharm Bull 42:368–370. https://doi.org/10.1248/cpb.42.368
Zhang G, Yang C, Liu E, Golen J, Rheingold AL (2014) Mild, green copper/4-dimethylaminopyridine catalysed aerobic oxidation of alcohols mediated by nitroxyl radicals in water. RSC Adv 4:61907–61911. https://doi.org/10.1039/c4ra13929a
Acknowledgements
The authors would like to thank Grant agencies of the Slovak Republic (VEGA 1/0639/18, APVV-18-0016) are gratefully acknowledged for their financial support. This article was created with the support of the MŠVVaŠ of the Slovak Republic within the Research and Development Operation Program for the project “University Science Park of STU Bratislava” (ITMS project no. 26240220084) cofounded by the European Regional Development Fund.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ondrejovič, G., Moncol, J. & Koman, M. The crystal structure of tetrameric copper(II) complexes, Hirshfeld surface analysis, and vector analyses of Cu4OCl6L4 complexes with N-donor ligands. Chem. Pap. 74, 3755–3766 (2020). https://doi.org/10.1007/s11696-020-01257-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-020-01257-4