Abstract
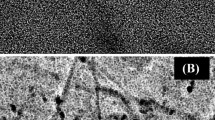
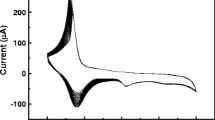
In the present paper, highly sensitive non-enzymatic sensor was developed by simple method based on direct deposition of gold particles on polyvinylferrocene matrix modified Pt electrode (Au/PVF/Pt) for detection of glucose. The gold particles were generated from potassium tetrachloroaurate (III) (KAuCl4) solution based on optimization of concentration of the solution and growth time of Au particles. The electrochemical characterization and electrocatalytic activities of the proposed sensor were studied with the help of cyclic voltammetry and chronoamperometry techniques. The Au/PVF/Pt sensor was evaluated on analytical parameters such as sensitivity, selectivity, stability, detection limits, repeatability, and reproducibility. As gold particles provide active sites towards oxidation of glucose, two wide linear ranges, 10–80 μM and 100–6 mM with ultra-sensitivities of 3236.3 and 730.3 μA mM−1 cm−2 respectively, were observed. Furthermore, limit of detection was calculated as 0.068 mM with fast response time of 2 s. Additionally, the sensor showed good repeatability and reproducibility with RSD of 3.03 and 2.14% respectively. The as-prepared Au/PVF/Pt sensor was able to show excellent stability and selectivity to glucose exclusively in the presence of other interfering species. Finally, the Au/PVF/Pt sensor could successfully detect glucose in various boxed juices samples. The results obtained from our method were compared to the commercial glucose meter. Hence, the applicability of Au/PVF/Pt sensor can be promising for determination of glucose in various fields such as food, biotechnology, and clinical diagnosis.











Similar content being viewed by others
References
Arjona N, Trejo G, Ledesma-García J, Arriaga LG, Guerra-Balcázar M (2016) An electrokinetic-combined electrochemical study of the glucose electro-oxidation reaction: effect of gold surface energy. RSC Adv 6:15630–15638
Aydın G, Çelebi SS, Özyörük H, Yıldız A (2002) Amperometric enzyme electrode for l(+)-lactate determination using immobilized l(+)-lactate oxidase in poly(vinylferrocenium) film. Sens Actuators B Chem 87:8–12
Bruckenstein S, Pater E, Hillman AR (2000) Open circuit reactions complicating the electroprecipitation of poly(vinylferricinium) films from methylene chloride. Anal Chem 72:1598–1603
Chaiyo S, Mehmeti E, Siangproh W, Long Hoang T, Phong Nguyen H, Chailapakul O, Kalcher K (2018) Non-enzymatic electrochemical detection of glucose with a disposable paper-based sensor using a cobalt phthalocyanine–ionic liquid–grapheme composite. Biosens Bioelectron 102:113–120
Chang G, Shu H, Ji K, Oyama M, Liu X, He Y (2014) Gold nanoparticles directly modified glassy carbon electrode for non-enzymatic detection of glucose. Appl Surf Sci 288:524–529
Chen J, Zheng J (2015) A highly sensitive non-enzymatic glucose sensor based on tremella-like Ni(OH)2 and Au nanohybrid films. J Electroanal Chem 749:83–88
Cherevko S, Chung CH (2009) Gold nanowire array electrode for non-enzymatic voltammetric and amperometric glucose detection. Sens Actuators B Chem 142:216–223
Çiftçi H, Alver E, Çelik F, Metin AÜ, Tamer U (2016) Non-enzymatic sensing of glucose using a glassy carbon electrode modified with gold nanoparticles coated with polyethyleneimine and 3-aminophenylboronic acid. Microchim Acta 183:1479–1486
Deng CY, Chen JH, Chen XL, Mao CH, Nie LH, Yao SZ (2008) Direct electrochemistry of glucose oxidase and biosensing for glucose based on boron-doped carbon nanotubes modified electrode. Biosens Bioelectron 23:1272–1277. https://doi.org/10.1016/j.bios.2007.11.009
Dolgin E (2012) Managed by machine. Nature 485:S6–S8
El Khatib KM, Abdel Hameed RM (2011) Development of Cu2O/carbon vulcan XC-72 as non-enzymatic sensor for glucose determination. Biosens Bioelectron 26:3542–3548
El-Ads EH, Galal A, Atta NF (2015) Electrochemistry of glucose at gold nanoparticles modified graphite/SrPdO3 electrode—towards a novel non-enzymatic glucose sensor. J Electroanal Chem 749:42–52
Fu S, Fan G, Yang L, Li F (2015) Non-enzymatic glucose sensor based on Au nanoparticles decorated ternary Ni-Al layered double hydroxide/single-walled carbon nanotubes/graphene nanocomposite. Electrochim Acta 152:146–154
Guo MM, Wang PS, Zhou CH, Xia Y, Huang W, Li Z (2014) An ultrasensitive non-enzymatic amperometric glucose sensor based on a Cu-coated nanoporous gold film involving co-mediating. Sens Actuators B Chem 203:388–395
Hasan MdM, Hossain MdE, Mamun MA, Ehsan MQ (2012) Study of redox behavior of Cd(II) and interaction of Cd(II) with proline in the aqueous medium using cyclic voltammetry. J Saudi Chem Soc 16:145–151
Heli H, Amirizadeh O (2016) Non-enzymatic glucose biosensor based on hyperbranched pine-like gold nanostructure. Mater Sci Eng, C 63:150–154
Ingel JD, Crouch SR (1998) Spectrochemical analysis. Prentice-Hall inc, Englewood Cliffs, p 173
Ismail NS, Le QH, Yoshikawa H, Saito M, Tamiya E (2014) Development of non-enzymatic electrochemical glucose sensor based on graphene oxide nanoribbon—gold nanoparticle hybrid. Electrochim Acta 146:98–105
Kangkamano T, Numnuam A, Limbut W, Kanatharana P, Thavarungkul P (2017) Chitosan cryogel with embedded gold nanoparticles decorated multiwalled carbon nanotubes modified electrode for highly sensitive flow based non-enzymatic glucose sensor. Sens Actuators B Chem 246:854–863
Karnes HT, Shiu G, Shah VP (1991) Validation of bioanalytical methods. Pharm Res 8:421–426
Kavanoz M, Kamış H, Yildiz A (2004) Anodic stripping voltammetric determination of gold on polyvinylferrocene coated glassy carbon electrode. Turkish J Chem 28:287–297
Kuralay F, zyörük HÖ, Yıldız A (2006) Amperometric enzyme electrode for urea determination using immobilized urease in poly(vinylferrocenium) film. Sens Actuators B Chem 114:500–506
Liu L, Chen Y, Lv H, Wang G, Hu X, Wang C (2015) Construction of a non-enzymatic glucose sensor based on copper nanoparticles/poly(o-phenylenediamine) nanocomposites. J Solid State Electrochem 19:731–738
Özer BC, Özyörük H, Çelebi SS, Yıldız A (2007) Amperometric enzyme electrode for free cholesterol determination prepared with cholesterol oxidase immobilized in poly(vinylferrocenium) film. Enzyme Microbial Technol 40:262–265
Rezaei B, Esfahani MH, Ensafi AA (2016) Modified Au nanoparticles/imprinted sol-gel/multiwall carbon nanotubes pencil graphite electrode as a selective electrochemical sensor for papaverine determination. IEEE Sens J 16:7037–7044
Shen C, Su J, Li X, Luo J, Yang M (2015) Electrochemical sensing platform based on Pd–Au bimetallic cluster for non-enzymatic detection of glucose. Sens Actuators B Chem 209:695–700
Shu H, Cao L, Chang G, He H, Zhang Y, He Y (2014) Direct electrodeposition of gold nanostructures onto glassy carbon electrodes for non-enzymatic detection of glucose. Electrochim Acta 132:524–532
Sulak MT, Gökdoğan Ö, Gülce A, Gülce H (2006) Amperometric glucose biosensor based on gold-deposited polyvinylferrocene film on Pt electrode. Biosens Bioelectron 21:1719–1726
Thanh TD, Balamurugan J, Yeon Hwang J, Kim NH, Lee JH (2016) In situ synthesis of graphene-encapsulated gold nanoparticle hybrid electrodes for non-enzymatic glucose sensing. Carbon 98:90–98
Tian K, Prestgard M, Tiwari A (2014) A review of recent advances in non enzymatic glucose sensors. Mater Sci Eng, C 41:100–118
Toghill KE, Compton RG (2010) Electrochemical non-enzymatic glucose sensors: a perspective and an evaluation. Int J Electrochem Sci 5:1246–1301
Wang Q, Cui X, Chen J, Zheng X, Liu C, Xue T, Wang H, Jin Z, Qiao L, Zheng W (2012) Well-dispersed palladium nanoparticles on graphene oxide as a non-enzymatic glucose sensor. RSC Adv 2:6245–6249
Wang G, He X, Wang L, Gu A, Huang Y, Fang B, Geng B, Zhang X (2013a) Non-enzymatic electrochemical sensing of glucose. Microchim Acta 180:161–186
Wang Z, Lei H, Feng L (2013b) A facile channel for d-glucose detection in aqueous solution. Spectrochim Acta A 114:293–297
Xiao F, Zhao F, Mei D, Mo Z, Zeng B (2009) Nonenzymatic glucose sensor based on ultrasonic-electrodeposition of bimetallic PtM (M = Ru, Pd and Au) nanoparticles on carbon nanotubes–ionic liquid composite film. Biosens Bioelectron 24:3481–3486
Xiaomei C, Genghuang W, Zhixiong C, Munetaka O, Xi C (2014) Advances in enzyme-free electrochemical sensors for hydrogen peroxide, glucose, and uric acid. Microchim Acta 181:689–705
Xu M, Song Y, Ye Y, Gong C, Shen Y, Wang L, Wang L (2017) A novel flexible electrochemical glucose sensor based on gold nanoparticles/polyaniline arrays/carbon cloth electrode. Sens Actuators B Chem 252:1187–1193
Yu L, Sathe M, Zeng XJ (2005) EQCM study of the redox processes of polyvinylferrocene film in L-glutamine solution. J Electrochem Soc 152:E357–E363
Acknowledgements
We gratefully acknowledge financial support from the University of Wisconsin Oshkosh Faculty Development Program. We would like to thank Dr. Todd Kostman of Biology Department of UW Oshkosh for conducting SEM work. Also, we are thankful to Dean, Faculty of Engineering and Technology, Manav Rachna International Institute of Research and Studies (formerly as MRIU), Faridabad, for his kind support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Malhotra, S., Tang, Y. & Varshney, P.K. Non-enzymatic glucose sensor of high sensitivity fabricated with direct deposition of Au particles on polyvinylferrocene film modified Pt electrode. Chem. Pap. 73, 1987–1996 (2019). https://doi.org/10.1007/s11696-019-00752-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-019-00752-7