Abstract
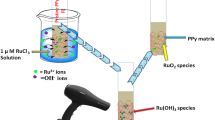
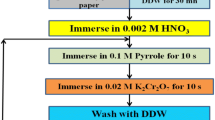
Present work is a study of effect of precursor bath temperature on the surface morphology and electrochemical performance of PPy:FeOOH hybrid flexible electrodes (HFEs). SILAR synthesis has been carried out using aqueous solutions of 0.1 M pyrrole dissolved in 0.5 M H2SO4 and 0.1 M Fe(NO3)3. XRD patterns substantiate the formation of tetragonal FeOOH in the hybrid. It was found that the average crystallite size goes on increasing from 22.81 through 24.63 nm as the synthesis bath temperature goes on increasing from 278.15 through 315.15 K. Characteristic peak at 1559 cm−1 in FTIR spectra is due to the pyrrole ring vibration due to formation of PPy. This confirms the formation of PPy:FeOOH hybrid. The scanning electron microscopic images of the HFEs exhibit morphological transitions from mud-like to globular array and finally to randomly arranged interconnected tube-like morphology. The observed maximum specific capacitance in 0.5 M H2SO4 was 980.34 F g−1 at the voltage scan rate of 2 mV s−1. HFEs exhibit prolonged discharge. IR drop (potential drop across internal resistance) increases with current density. Maximum value of specific capacitance as calculated by galvanostatic charge–discharge analysis is 969.54 Fg−1, which closely matches to that given by the cyclic voltammetry producing nearly same current. The equivalent series resistance (ESR), charge transfer resistance (Rct) and Warburg impedance (Rw) as evaluated from the Nyquist plot were ~ 5.31 Ω, ~ 4.30 Ω and ~ 3.70 Ω, respectively.







Similar content being viewed by others
References
Ambade RB, Ambade SB, Salunkhe RR, Malgras V, Jin S-H, Yamauchi Y, Lee S-H (2018) Flexible wire shaped all solid state supercapacitors based on facile electropoly densityon of polythiophene with ultra high energy density. J Mater Chem A 4(19):7406–7415. https://doi.org/10.1002/adfm.201703949
Choudhari S, Bhattacharya D, Yu JS (2013) 1-Dimensional porous α Fe2O3 nanorods as high performance electrode material for supercapacitor. RSC Adv 3:25120. https://doi.org/10.1039/c3ra44159h
Davies A, Audette P, Farrow B, Hassan F, Chen Z, Choi JY, Yu A (2011) Graphene-based flexible supercapacitors: pulse-electropolymerization of polypyrrole on free-standing graphene films. J Phys Chem C 115:17612
Gong X, Li S, Lee PS (2017) Fiber asymmetric supercapacitor based on FeOOH/PPy on carbon fibers as anode electrode with high volumetric energy density for wearable applications. Nanoscale. https://doi.org/10.1039/x0xx00000x
Hammar A, Venet P, Lallemand R, Coquery G, Rojat G (2012) Study of accelerated aging of supercapacitors for transport applications. IEEE Trans Industr Electron 57:12
Han G, Liu Y, Zhang L, Kan E, Zhang S, Tang J, Tang W (2012) MnO2 nanorods intercalating graphene oxide/polyaniline ternary composites for robust high-performance supercapacitors. Sci Rep 4:4824. https://doi.org/10.1038/srep04824
Li Xinran, Xiao Xiao, Quing Li J, Wei H, Xue H Pang (2018) Metal phosphate based materials for high performance supercapacitors. Inorg Chem Front 5:11–28
Liang Wenbin, Lei Junting, Martin Charles R (1992) Effect of synthesis temperature on the structure, doping level and charge-transport properties of polypyrrole. Synth Met 52:227–239
Lindsey SE, Street GB (1984/85) Conductive composites from poly (vinyl alcohol) and polypyrrole. Synth Met 10(1):67–69
Lu Y, Li B, Zheng S, Xu Y, Xue H, Pang H (2017) Synthesis and energy storage applications of MxSy (M = Cu, Ag, Au) and their composites: rechargeable batteries and supercapacitors. Adv Func Mater 27(44):1703949
Salunkhe RR, Tiang J, Kobayashi N, Kim J, Ide Yusuke, Tominaka S, Kim JH, Yamauchi Y (2016) Ultrahigh performance supercapacitors utilizing core-shell nanoarchitectures from metal-organic framework derived nanoparticles carbon and conducting polymers. Chem Sci 7(9):5704–5713
Salunkhe RR, Kaneti YV, Yamauchi Y (2017) Metal organic framework derived nanoporous metal oxides towards supercapacitor applications: progress and prospects. ACS Nano 11:5293–5308
Shi C, Zhitomirsky I (2010) Electrodeposition and capacitive behavior of films for electrodes of electrochemical supercapacitors. Nanoscale Res Lett 5:518–523
Shinde S, Gund GS, Kumbhar VS, Patil BH, Lokhande CD (2013) Novel chemical synthesis of polypyrrole thin film electrodes for supercapacitor application. Eur Polym J 49:3734–3739
Shinde SS, Gund GS, Dubal DP, Jambure SB, Lokhande CD (2014) Morphological modulation of polypyrrole thin films through oxidizing agents and their concurrent effect on supercapacitor performance. Electrochim Acta 119:1–10. https://doi.org/10.1016/j.electacta.2013.10.174
Snook GA, Best AS (2011) Review on conducting polymer based supercapacitor devices and electrodes. J Power Sources 196:1–12
Tang J, Yamauchi Y (2016) Carbon materials: MOF morphologies in control. Nat Chem 8(7):638
Thakur AV, Lokhande BJ (2016) Effect of dip time on the electrochemical behavior of PPy-Cu(OH)2 hybrid electrodes synthesized using pyrrole and Cu(SO)4. e-Polymer. https://doi.org/10.1515/epoly-2016-0160
Thakur AV, Lokhande BJ (2017a) Dip time dependent SILAR synthesis and electrochemical study of highly flexible PPy-Cu(OH)2 hybrid electrodes for supercapacitors. J Solid State Electrochem. https://doi.org/10.1007/s10008-016-3502-2
Thakur AV, Lokhande BJ (2017b) C10H8N2-PPy hybrid flexible electrode: SILAR synthesis and electrochemical study. J Mater Sci Mater Electron. https://doi.org/10.1007/s10854-017-8074-0
Thakur AV, Lokhande BJ (2017c) Electrolytic anion affected charge storage mechanisms of Fe3O4 flexible thin film electrode in KCl and KOH: a comparative study by cyclic voltammetry and galvanostatic charge–discharge. Mater Electron J Mater Sci. https://doi.org/10.1007/s10854-017-6980-9
Thakur AV, Lokhande BJ (2018a) Morphological modification for optimum electrochemical performance of highly pristine polypyrrole flexible electrodes via SILAR immersion time and fabrication of solid state symmetric device. Port Electrochim Acta 36(6):377–392
Thakur AV, Lokhande BJ (2018b) Source molarity affected surface morphological and electrochemical transitions in binder-free FeO(OH) flexible electrodes and fabrication of symmetric supercapacitive device. Chem Pap. https://doi.org/10.1007/s11696-018-0383-0
Torabi M, Soltani M, Sadrnezhaad SK (2014) Conducting polymer composites: I. Surface-induced polymerization of pyrrole on iron(III) and cerium(IV) oxide particles. J New Mater Electrochem Syst 17:129–132. https://doi.org/10.1016/0021-9797(91)90234-Y
Wang H, Hao Q, Yang X, Lu L, Wang X (2010) Effect of graphene oxide on the properties of its composite with polyaniline. Appl Mater Interface 2:821–828
Acknowledgements
Authors are thankful for Solapur University for the provision of DRF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On the behalf of all authors, the corresponding author states that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Thakur, A.V., Lokhande, B.J. Effect of precursor bath temperature on the morphology and electrochemical performance of SILAR-synthesized PPy:FeOOH hybrid flexible electrodes. Chem. Pap. 73, 833–841 (2019). https://doi.org/10.1007/s11696-018-0644-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-018-0644-y