Abstract
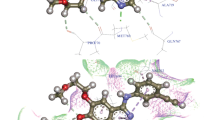
Investigations in the discovery of tyrosinase enzyme inhibitors have the potential to design novel anti-cancer drugs. A variety of novel bivalent pyrazolinyl triazoles 5–7 were synthesized and identified. The bis-acetyl triazole 3 was accomplished via two-step process first by preparation of diazide 2 followed its coupling reaction with acetylacetone in basic medium. The Claisen–Schmidt condensation reaction of 3 with two moles equivalent of benzaldehyde produces the corresponding bis-α, β-unsaturated ketone 4. Treatment of Schiff base 4 with excess hydrazine hydrate in either acetic acid or ethanol/DMF afforded the bis-N-acetylpyrazline 5 or bis-pyrazoline 6, respectively. Finally, treatment of 6 with two derivatives of isothiocyanate analog gives the bis-N-thioamide pyrazolines 7a and 7b. The synthesized compounds were evaluated as anti-tumor candidates against three human cancer cell lines (MCF-7, HepG2, and HCT-116). The bioscreening evaluation showed that compounds 7a and 6 had a significant antineoplastic potencies (IC50: 32.26 and 57.06 µg/mL) against breast MCF-7 and hepatic HepG2 cancerous cell lines, respectively, in relative to the standard drug, 5-fluorouracil. Molecular docking studies of the synthesized compounds were investigated as VEGFR2 TK inhibitors.
Graphical Abstract








Similar content being viewed by others
References
Abdou WM, Bekheit MS (2015) One pot three-component synthesis of peptidomimics for investigation of antibacterial and antineoplastic properties. Arab J Chem. https://doi.org/10.1016/j.arabjc.2015.04.014
Abd-Rabou AA (2017) Calcium, a cell cycle commander drives colon cancer cell diffpoptosis. Indian J Clin Biochem 32:9–18. https://doi.org/10.1007/s12291-016-0562-0
Abd-Rabou AA, Ahmed HH (2017) CS-PEG decorated PLGA nano-prototype for delivery of bioactive compounds: a novel approach for induction of apoptosis in HepG2 cell line. Adv Med Sci 62:357–367. https://doi.org/10.1016/j.advms.2017.01.003
Abd-Rabou AA, Zoheir K, Ahmed HH (2012) Potential impact of curcumin and taurine on human hepatoma cells using Huh-7 cell line. Clin Biochem 45:1519–1521. https://doi.org/10.1016/j.clinbiochem.2012.06.032
Abd-Rabou AA, Zoheir KMA, Kishta MS, Shalby AB, Ezzo MI (2016) Nano-micelle of moringa oleifera seed oil triggers mitochondrial cancer cell apoptosis. Asian Pac J Cancer Prev 17:4929–4933. https://doi.org/10.22034/APJCP.2016.17.11.4929
Ahmed HH, Abd-Rabou AA, Hassan AZ, Kotob SE (2015) Phytochemical analysis and anti-cancer investigation of boswellia serrata bioactive constituents in vitro. Asian Pac J Cancer Prev 16:7179–7188. https://doi.org/10.7314/APJCP.2015.16.16.7179
Amin KM, Abou-Seri SM, Awadallah FM, Eissa AAM, Hassan GS, Abdulla MM (2015) Synthesis and anticancer activity of some 8-substituted-7-methoxy-2H-chromen-2-one derivatives toward hepatocellular carcinoma HepG2 cells. Eur J Med Chem 90:221–231. https://doi.org/10.1016/j.ejmech.2014.11.027
Baashen MA, Abdel-Wahab BF, El-Hiti GA (2016) Syntheses of triazoloquinoxalines. Heterocycles 92:1931–1952. https://doi.org/10.3987/REV-16-847
Bano S, Javed K, Ahmad S, Rathish IG, Singh S, Alam MS (2011) Synthesis and biological evaluation of some new 2-pyrazolines bearing benzene sulfonamide moiety as potential anti-inflammatory and anti-cancer agents. Eur J Med Chem 46:5763–5768. https://doi.org/10.1016/j.ejmech.2011.08.015
Bekheit MS, Kamel AA (2017) Multi-Component reactions in preparation of α- and β- substituted phosphonates. Curr Org Chem 21:923–938. https://doi.org/10.2174/1385272821666170113114456
Bekheit MS, Farahat AA, Abdel-Wahab BF (2016) Synthetic routes to thiazoloquinazoline. Chem Heterocycl Compd 52:766–772. https://doi.org/10.1007/s10593-016-1961-0
Berque-Bestel I, Lezoualc’h F, Jockers R (2008) Bivalent ligands as specific pharmacological tools for G protein-coupled receptor dimers. Curr Drug Discov Technol 5:312–318. https://doi.org/10.2174/157016308786733591
Bhuva HHA, Kini SG (2010) Synthesis, anticancer activity and docking of some substituted benzothiazoles as tyrosine kinase inhibitors. J Mol Graph Model 29:32–37. https://doi.org/10.1016/j.jmgm.2010.04.003
Demchuk DV, Samet AV, Chernysheva NB, Ushkarov VI, Stashina GA, Konyushkin LD, Raihstat MM, Firgang SI (2014) Synthesis and antiproliferative activity of conformationally restricted 1,2,3-triazole analogues of combretastatins in the sea urchin embryo model and against human cancer cell line. Bioorg Med Chem 15:738–755. https://doi.org/10.1016/j.bmc.2013.12.015
George DJ (2007) Phase 2 studies of sunitinib and AG013736 in patients with cytokine-refractory renal cell carcinoma. Clin Cancer Res 13:753–757. https://doi.org/10.1158/1078-0432.CCR-06-2044
Gfeller D, Grosdidier A, Wirth M, Daina A, Michielin O, Zoete V (2014) SwissTargetPrediction: a web server for target prediction of bioactive small molecules. Nucleic Acids Res 42:W32–W38. https://doi.org/10.1093/nar/gku293
Gupta D, Jain DKJ (2015) Synthesis, antifungal and antibacterial activity of novel 1,2,4-triazole derivative. J Adv Pharm Technol Res 6:141–146. https://doi.org/10.4103/2231-4040.161515
Hassan AS, Hafez TS, Ali MM, Khatab TK (2016) Design, synthesis and cytotoxic activity of some new pyrazolines bearing benzofuran and pyrazole moieties. Res J Pharm Biol Chem Sci 7:417–429
Hiller C, Kuhhorn J, Gmeiner PJ (2013) Class A G-Protein-Coupled receptor (GPCR) dimers and bivalent ligands. J Med Chem 56:6542–6559. https://doi.org/10.1021/jm4004335
Kharb R, Yar MS, Sharma PC (2011) Recent advances and future perspectives of triazole analogs as promising antiviral agents. Mini Rev Med Chem 11:84–96. https://doi.org/10.2174/138955711793564051
Khaybullin RN, Zhang M, Fu J, Liang X, Li T, Katritzky AR, Okunieff P, Qi X (2014) Design and synthesis of isosteviol triazole conjugates for cancer therapy. Molecules 19:18676–18689. https://doi.org/10.3390/molecules191118676
Khazir J, Hyder I, Gayatri JL, Yandrati LP, Nalla N, Chasoo G, Mahajan A, Saxena AK, Alam MS, Qazi GN, Kumar HMS (2014) Design and synthesis of novel 1,2,3-triazole derivatives of coronopilin as anti-cancer compound. Eur J Med Chem 82:255–262. https://doi.org/10.1016/j.ejmech.2014.05.053
Lambert PA, Somers KD, Kohn EC, Perry RR (1997) Antiproliferative and antiinvasive effects of carboxyamido-triazole on breast cancer cell lines. Surgery 122:372–378
Li Z, Wan H, Shi Y, Ouyang P (2004) Personal experience with four kinds of chemical structure drawing software: review on ChemDraw, ChemWindow, ISIS/Draw, and ChemSketch. J Chem Inf Comput Sci 44:1886–1890. https://doi.org/10.1021/ci049794h
Li D, Wang X, Jia Y, Wang A, Wu Y (2012) Synthesis of conjugated hyperbranched polytriazoles containing truxene units by click polymerization. Chin J Chem 30:861–868. https://doi.org/10.1002/cjoc.201100339
Lv P-C, Li D-D, Li Q-S, Lu X, Z-p Xiao, Zhu H-L (2011) Synthesis, molecular docking and evaluation of thiazolyl-pyrazoline derivatives as EGFR TK inhibitors and potential anticancer agents. Bioorg Med Chem Lett 21:5374–5377. https://doi.org/10.1016/j.bmcl.2011.07.010
Marella A, Ali R, Alam T, Saha R, Tanwar O, Akhter M, Shaquiquzzaman M, Alam MM (2013) Pyrazolines: a biological review. Mini Rev Med Chem 13:921–931. https://doi.org/10.2174/1389557511313060012
Metwally MA, Abdel-Wahab BF, El-Hiti GA (2010) 2-Acetylbenzofurans: synthesis, reactions and application. Curr Org Chem 14:48–64. https://doi.org/10.2174/138527210790226401
Na JI, Na JY, Choi WY, Lee MC, Park MS, Choi KH, Lee JK, Kim KT, Park JT, Kim HS (2015) The HIF-1 inhibitor YC-1 decreases reactive astrocyte formation in a rodent ischemia model. Am J Transl Res 7:751–760
Nussbaumer S, Bonnabry P, Veuthey J-L, Fleury-Souverain S (2011) Analysis of anticancer drugs: a review. Talanta 85:2265–2289. https://doi.org/10.1016/j.talanta.2011.08.034
Paprocka R, Wiese M, Eljaszewicz A, Helmin-Basa A, Gzella A, Modzelewska-Banachiewicz B, Michalkiewicz J (2015) Synthesis and anti-inflammatory activity of new 1,2,4-triazole derivative. Bioorg Med Chem Lett 25:2664–2667. https://doi.org/10.1016/j.bmcl.2015.04.079
Parikh AA, Ellis LM (2004) The vascular endothelial growth factor family and its receptors. Hematol Oncol Clin North Am 18:951–971. https://doi.org/10.1016/j.hoc.2004.06.004
Pedretti A, Villa L, Vistoli g (2004) VEGA-An open platform to develop chemo-bio-informatics applications, using plug-in architecture and script programming. J Comput Aided Mol Des 18:167–173. https://doi.org/10.1023/B:JCAM.0000035186.90683.f2
Pokhodylo N, Shyyka O, Matiychuk V (2013) Synthesis of 1,2,3-triazole derivatives and evaluation of their anticancer activity. Sci Pharm 81:663–676. https://doi.org/10.3797/scipharm.1302-04
Powers DG, Casebier DS, Fokas D, Ryan WJ, Troth JR, Coffen DL (1998) Automated parallel synthesis of chalcone-based screening libraries. Tetrahedron 54:4085–4096. https://doi.org/10.1016/S0040-4020(98)00137-9
Qin H-L, Shang Z-P, Jantan I, Tan OU, Hussain MA, Sher M, Bukhari SNA (2015a) Molecular docking studies and biological evaluation of chalcone based pyrazolines as tyrosinase inhibitors and potential anticancer agents. RSC Adv 5:46330–46338. https://doi.org/10.1039/C5RA02995C
Qin Y-J, Li Y-J, Jiang A-Q, Yang M-R, Zhu Q-Z, Dong H, Zhu H-L (2015b) Design, synthesis and biological evaluation of novel pyrazoline-containing derivatives as potential tubulin assembling inhibitor. Eur J Med Chem 94:447–457. https://doi.org/10.1016/j.ejmech.2015.02.058
Rahman A, Siddiqui AA (2010) Pyrazoline derivatives: a worthy insight into the recent advances and potential pharmacological activities. Inter J Pharm Sci Drug Res 2:165–175
Ramaswamy B, Mrozek E, Kuebler JP, Bekaii-Saab T, Kraut EH (2011) Phase II trial of pyrazoloacridine (NSC#366140) in patients with metastatic breast cancer. Investig New Drugs 29:347–351. https://doi.org/10.1007/s10637-009-9338-1
Ranieri G, Mammi M, di Paola ED, Russo E, Gallelli L, Citraro R, Gadaleta CD, Marech I, Ammendola M, de Sarro G (2014) Pazopanib a tyrosine kinase inhibitor with strong anti-angiogenetic activity: a new treatment for metastatic soft tissue sarcom. Crit Rev Oncol Hematol 89:322–329. https://doi.org/10.1016/j.critrevonc.2013.08.012
Rathore P, Yaseen S, Ovais S, Bashir R, Yaseen R, Hameed AD, Samim M, Gupta R, Hussain F, Javed K (2014) Synthesis and evaluation of some new pyrazoline substituted benzenesulfonylureas as potential antiproliferative agent. Bioorg Med Chem Lett 24:1685–1691. https://doi.org/10.1016/j.bmcl.2014.02.059
Rebucci M, Michiels C (2013) Molecular aspects of cancer cell resistance to chemotherapy. Biochem Pharmacol 85:1219–1226. https://doi.org/10.1016/j.bcp.2013.02.017
Rosini M, Simoni E, Bartolini M, Soriano E, Marco-Contelles J, Andrisano V, Monti B, Windisch M, Hutter-Paier B, McClymont DW, Mellor LR, Bolognesi ML (2013) The bivalent ligand approach as a tool for improving the in vitro anti-alzheimer multitarget profile of dimebon. Chem Med Chem 8:1276–1281. https://doi.org/10.1002/cmdc.201300263
Shaaban MR, Mayhoub AS, Farag AM (2012) Recent advances in the therapeutic applications of pyrazoline. Expert Opin Ther Pat 22:253–291. https://doi.org/10.1517/13543776.2012.667403
Shin SY, Yoon H, Hwang D, Ahn S, Kim D-W, Koh D, Lee YH, Lim Y (2013) Benzochalcones bearing pyrazoline moieties show anti-colorectal cancer activities and selective inhibitory effects on aurora kinases. Bioorg Med Chem 21:7018–7024. https://doi.org/10.1016/j.bmc.2013.09.014
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. Cancer J Clin 66:7–30. https://doi.org/10.3322/caac.21332
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. Cancer J Clin 67:7–30. https://doi.org/10.3322/caac.21387
Trott A, Olson J (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreadin. J Comput Chem 31:455–461. https://doi.org/10.1002/jcc.21334
van Meerloo J, Kaspers GJ, Cloos J (2011) Cell sensitivity assays: the MTT assayCancer cell culture. Methods Mol Biol 731:237–245. https://doi.org/10.1007/978-1-61779-080-5_20
Varmus H (2006) The new era in cancer research. Science 312:1162–1165. https://doi.org/10.1126/science.1126758
Vatmurge NS, Hazra BG, Pore VS, Shirazi F, Chavan PS, Mv Deshpande (2018) Synthesis and antimicrobial activity of β-lactam–bile acid conjugates linked via triazole. Bioorg Med Chem Lett 18:2043–2047. https://doi.org/10.1016/j.bmcl.2008.01.102
Xu W, Pan Y, Wang H, Li H, Peng Q, Wei D, Chen C, Zheng J (2017) Synthesis and evaluation of new pyrazoline derivatives as potential anticancer agents in HepG2 cell line. Molecules 22:467–481. https://doi.org/10.3390/molecules22030467
Yusuf M, Jain P (2014) Synthetic and biological studies of pyrazolines and related heterocyclic compounds. Arab J Chem 7:553–596. https://doi.org/10.1016/j.arabjc.2011.09.013
Zhu SL, Wu Y, Liu CJ, Wei CY, Tao JC, Liu HM (2013) Design and stereoselective synthesis of novel isosteviol-fused pyrazolines and pyrazoles as potential anticancer agents. Eur J Med Chem 65:70–82. https://doi.org/10.1016/j.ejmech.2013.04.044
Zoheir KMA, Abd-Rabou AA, Harisa GI (2015) Gene expression of IQGAPs and Ras families in an experimental mouse model for hepatocellular carcinoma: a mechanistic study of cancer progression. Int J Clin Exp Pathol 8:8821–8831
Zoheir KMA, Abd-Rabou AA, Harisa GI, Kumar A, Ahmad SF, Ansari MA, Abd-Allah AR (2016) IQGAP1 gene silencing induces apoptosis and decreases the invasive capacity of human hepatocellular carcinoma. Tumor Biol 37:13927–13939. https://doi.org/10.1007/s13277-016-5283-8
Zwick E, Bange J, Ullrich A (2001) Receptor tyrosine kinase signalling as a target for cancer intervention strategies. Endocr Relat Cancer 8:161–173. https://doi.org/10.1677/erc.0.0080161
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abd-Rabou, A.A., Abdel-Wahab, B.F. & Bekheit, M.S. Synthesis, molecular docking, and evaluation of novel bivalent pyrazolinyl-1,2,3-triazoles as potential VEGFR TK inhibitors and anti-cancer agents. Chem. Pap. 72, 2225–2237 (2018). https://doi.org/10.1007/s11696-018-0451-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-018-0451-5