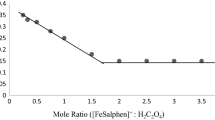
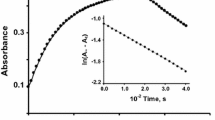
Abstract
Protonation plays an important role in the redox reactions. We observed this leading role during the reduction of [FeIII(phen)2(CN)2]+ by FcCOMe and FcCHOHMe. The kinetic data showed that the reaction(s) followed a complex kinetics due to the formation of protonated acetylferrocene (FcC+OHMe), and or, protonated α-methylferrocenemethanol (FcCHO+H2Me) in aqueous dioxane (80% v/v). Our results helped us to conclude that the reactions were completed in three phases. An overall zeroth order was observed in the first phase of the reactions. In the second phase, the kinetic data showed an overall second order reaction. The third phase was a complex phase where the rate of redox reactions and the insolubility of the neutral product ([FeII(phen)2(CN)2]) competed with each other. We studied the effect of different factors to identify the reacting entities, which take part in the rate-determining step of each reaction in the second phase. Consequently, we determined the effects of selected factors upon the observed pseudo-first order rate constant(s) (k′ obs) of each reaction. The value of k′ obs increased upon addition of protons in the reaction mixture in case of FcCOMe, and it decreased during the oxidation of FcCHOHMe. Meanwhile, upon enhancing the ionic strength, we observed an increase in k′ obs for FcCOMe, and no change in the value of k′ obs during the reaction of FcCHOHMe. However, a decrease in k′ obs was noticed upon increasing the dielectric constant of the reaction mixture when the reductant was FcCOMe, and no effect was observed in case of FcCHOHMe. Together, these results suggested oxidation of FcC+OHMe and FcCHOHMe in the slow-step, and FcCOMe and FcCHO+H2Me during the fast-step. We refined our results by estimating the thermodynamic parameters of activation. The low values of activation energy and enthalpy of activation confirmed that the reduction of [FeIII(phen)2(CN)2]+ hardly depends upon temperature when the reducing agent is FcCOMe. The outcomes justified that the rate of reaction depends upon the unsaturated FcC+OHMe. This intermediate species contain a ‘carbonium ion’, which is very reactive and energetic. We obtained comparatively high values of the activation energy and enthalpy of activation for the reaction between [FeIII(phen)2(CN)2]+ and FcCHOHMe. The results show that FcCHOHMe is a saturated and stable compound that leads the slow-step and controls the rate of reaction.






Similar content being viewed by others
Change history
08 January 2018
The original article can be found online at https://doi.org/10.1007/s11696-017-0334-1.
References
Arnett EM, Bushick RD (1962) Quantitative estimates of the strong electron donor properties of metallocenyl nuclei. J Org Chem 27(1):111–115. https://doi.org/10.1021/jo01048a028
Baciocchi E, Floris B, Muraglia E (1993) Reactions of ferrocene and acetylferrocene with carbon-centered free radicals. J Org Chem 58(8):2013–2016. https://doi.org/10.1021/jo00060a011
Blake R, White KJ, Shute EA (1991) Mixed ligand complexes of iron with cyanide and phenanthroline as new probes of metalloprotein electron transfer reactivity. Analysis of reactions involving rusticyanin from Thiobacillus ferrooxidans. J Biol Chem 266(29):19203–19211. http://www.jbc.org/content/266/29/19203.full.pdf
Casas JS, Castaño MV, Cifuentes MC, García-Monteagudo JC, Sánchez A, Sordo J, Touceda A (2007) Interaction of organolead(iv) derivatives with formyl- and acetylferrocene thiosemicarbazones: coordination versus dephenylation or reductive elimination processes. J. Organometal Chem 692(11):2234–2244. https://doi.org/10.1016/j.jorganchem.2007.01.049
Connelly NG, Geiger WE (1996) Chemical redox agents for organometallic chemistry. Chem Rev 96(2):877–910. https://doi.org/10.1021/cr940053x
Floris B (2015) Ferrocene in agriculture: from agrochemicals and soil remediation to selective chemosensors. Chem Biol Technol Agric 2(1):15. https://doi.org/10.1186/s40538-015-0038-0
Gao Z-N, Ma J-F, Liu W-Y (2005a) Electrocatalytic oxidation of sulfite by acetylferrocene at glassy carbon electrode. Appl Organometal Chem 19(11):1149–1154. https://doi.org/10.1002/aoc.975
Gao Z-N, Zhang J, Liu W-Y (2005) Electrocatalytic oxidation of n-acetyl-l-cysteine by acetylferrocene at glassy carbon electrode. J Electroanal Chem 580(1):9–16. http://www.sciencedirect.com/science/article/B6TGB-4FXV79B-6/2/31261f9ef9b2fb7ce46753ffbffd8415
Jong S-J, Fang J-M, Lin C-H (1999) The reactions of acylferrocenes with samarium diiodide: reduction, deoxygenation, reductive coupling and rearrangement. J Organometal Chem 590(1):42–45. https://doi.org/10.1016/S0022-328X(99)00411-8
Khattak R (2011) Comparative kinetic study for the electron transfer reactions of some iron complexes. In: Department of Chemistry. University of Karachi, Karachi, p 501
Khattak R, Naqvi II, Farrukh MA (2008) Kinetics and mechanism of the oxidation of a ferrous complex with an α, α′-diimine chelate ligand by ceric sulfate in aqueous acidic medium by UV-Vis absorption spectroscopy. J Iran Chem Soc 5(4):631–640. https://doi.org/10.1007/BF03246144
Khattak R, Naqvi II, Summer S, Sayed M (2016) Mechanism of the oxidation of 1-(ferrocenyl)-ethanone/ethanol by dicyanobis(phenanthroline)iron(iii). Arab J Chem. https://doi.org/10.1016/j.arabjc.2016.05.007
Matsumoto M, Tarumi T, Sugimoto K-I, Kagayama N, Funahashi S, Takagi HD (1997) Oxidation reaction of l-ascorbic acid by dicyanobis(1,10-phenanthroline)iron(iii) in dimethyl sulfoxide at elevated pressure: evidence for adiabatic electron transfer. Inorg Chim Acta 255:81–85. https://doi.org/10.1016/S0020-1693(96)05342-X
Osella D, Ferrali M, Zanello P, Laschi F, Fontani M, Nervi C, Cavigiolio G (2000) On the mechanism of the antitumour activity of ferrocenium derivatives. Inorg Chim Acta 306:42–48. https://doi.org/10.1016/S0020-1693(00)00147-X
Patra M, Gasser G (2017) The medicinal chemistry of ferrocene and its derivatives. Nat Rev Chem 1:0066. https://doi.org/10.1038/s41570-017-0066
Pelizzetti E, Mentasti E, Pramauro E (1978) Outer-sphere oxidation of ascorbic acid. Inorg Chem 17(5):1181–1186. https://doi.org/10.1021/ic50183a018
Pladziewicz JR, Espenson JH (1973) Kinetics and mechanisms of some electron transfer reactions of ferrocenes. J Am Chem Soc 95(1):56–63. https://doi.org/10.1021/ja00782a011
Quirk PF, Kratochvil B (1970) Determination of ferrocene derivatives by oxidation with copper(ii) in acetonitrile. Anal Chem 42(4):535–536. https://doi.org/10.1021/ac60286a035
Realista S, Quintal S, Martinho PN, Melato AI, Gil A, Esteves T, Carvalho MD, Ferreira LP, Vaz PD, Calhorda MJ (2017) Electrochemical studies and potential anticancer activity in ferrocene derivatives. J Coord Chem 70(2):314–327. https://doi.org/10.1080/00958972.2016.1257125
Rubalcava HE, Thomson JB (1963) A spectroscopic study of the protonation of acetylferrocenes. Hydrogen-deuterium exchange in the conjugate acids. J Phys Chem 67(2):310–313. https://doi.org/10.1021/j100796a023
Saleem M, Yu H, Wang L, Zain ul A, Khalid H, Akram M, Abbasi NM, Huang J (2015) Review on synthesis of ferrocene-based redox polymers and derivatives and their application in glucose sensing. Analyt Chim Acta 876(Supplement C):9–25. https://doi.org/10.1016/j.aca.2015.01.012
Sasaki Y, Pittman CU (1973) Acid-catalyzed reaction of acetylferrocene with triethyl orthoformate. J Org Chem 38(21):3723–3726. https://doi.org/10.1021/jo00961a014
Schilt AA (1960) Mixed ligand complexes of iron(ii) and (iii) with cyanide and aromatic di-imines. J Am Chem Soc 82(12):3000–3005. https://doi.org/10.1021/ja01497a007
Shago RF, Swarts JC, Kreft E, Rensburg CEJV (2007) Antineoplastic activity of a series of ferrocene-containing alcohols. Anticancer Res 27:3431–3434. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.1022.7131&rep=rep1&type=pdf
Summer S, Naqvi II, Khattak R, Gulzar S, Reyaz F (2016) Kinetics and mechanism of [fe (bipy)3]2+ and [bro −3 ] system in aqueous acidic medium. J Chem Soc Pak 38(03):384–389. http://www.jcsp.org.pk/ViewByVolume.aspx?v=1209&i=VOLUME%2038,%20NO3,%20JUNE-2016
Takagi HD, Kagayama N, Matsumoto M, Tarumi T, Funahashi S (1995) Mechanistic study of oxidation reactions of hydroquinone, catechol and l-ascorbic acid by dicyanobis(1,10-phenanthroline)iron(iii) in dimethyl sulfoxide. J Mol Liq 65–66:277–280. https://doi.org/10.1016/0167-7322(95)00827-0
Xu X, Nolan SP, Cole RB (1994) Electrochemical oxidation and nucleophilic addition reactions of metallocenes in electrospray mass spectrometry. Anal Chem 66(1):119–125. https://doi.org/10.1021/ac00073a021
Acknowledgements
The authors are indebted to the HEJ Research Institute of Chemistry, University of Karachi, Pakistan, for providing analytical services to carry out IR and microanalytical characterization of the synthesized compound(s).
Author information
Authors and Affiliations
Corresponding author
Additional information
This article belongs to the “Coordination and Bioinorganic Conference”.
A correction to this article is available online at https://doi.org/10.1007/s11696-017-0370-x.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khattak, R., Nazir, M., Summer, S. et al. Thermodynamic aspect: kinetics of the reduction of dicyanobis(phen)iron(III) by acetylferrocene and methylferrocenemethanol. Chem. Pap. 72, 883–893 (2018). https://doi.org/10.1007/s11696-017-0334-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-017-0334-1