Abstract
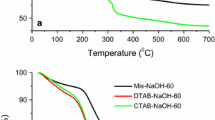
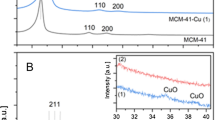
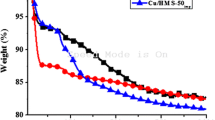
Mesoporous silica Si-MCM-41 was prepared by hydrothermal method using TEOS and CTAB as the source of silica and structuring agent, respectively. The surface of the as-synthesized material was treated using HCl/ETOH solvent to remove the CTA surfactant instead of using the calcination. Characterization of the catalysts was performed using X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), nitrogen sorption at 77 K, scanning and transmission electronic microscopy (SEM, TEM), and thermogravimetric analysis TGA. The catalytic properties of the prepared materials in the condensation of acetophenone with ethyl cyanoacetate were studied. The effects of the catalyst type, Si/Al ratio, reaction kinetics, and reaction temperature were also investigated to find an optimal parameter. The results show that an interesting yield was obtained (about 96%) in a short reaction time; it is found that the yields of products depend not only on the amount of surfactant inside the mesopores but also on the Si/Al ratio. The catalyst reuse shows that this catalyst can be used up to five cycles, and at temperatures higher than 50 °C, the yield of products decreases due to the slight destruction of the catalyst as confirmed by the XRD analysis. Based on the results obtained, a possible mechanism of the condensation reaction of acetophenone was proposed.










Similar content being viewed by others
References
Ariapad A, Zanjanchi MA, Arvand M (2012) Efficient removal of anionic surfactant using partial template-containing MCM-41. Desalination 284:142–149. doi:10.1016/j.desal.2011.08.048
Barrett EP, Joyner LG, Halenda PH (1951) The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J Am Chem Soc 73:373–380. doi:10.1021/ja01145a126
Beck JS, Vartuli JC, Roth WJ, Leonowicz ME, Kresge CT, Schmitt KD, Chu CTW, Olson DH, Sheppard EW, Mc Cullen SB, Higgins JB, Schlenker JL (1992) A new family of mesoporous molecular-sieves. Prepared with liquid-crystal templates. J Am Chem Soc 114:10834–10843. doi:10.1021/ja00053a020
Berquier JM, Teyssedre L, Jacquiod C (1998) Synthesis of transparent mesoporous and mesostructured thin silica films. J Sol-Gel Sci Technol 13:739–742. doi:10.1023/A:1008609525830
Boukoussa B, Sebih F, Hamacha R, Bellahouel S, Derdour A, Bengueddach A (2015a) Regioselective acylation of methyl-a-d-glucopyranoside with different acylating agents catalysed by micro/mesoporous materials. Res Chem Intermed 41:2221–2233. doi:10.1007/s11164-013-1340-8
Boukoussa B, Aouad N, Hamacha R, Bengueddach A (2015b) Key factor affecting the structural and textural properties of ZSM-5/MCM-41 composite. J Phys Chem Solids 78:78–83. doi:10.1016/j.jpcs.2014.11.006
Boukoussa B, Zeghada S, Bentabed Ababsa G, Hamacha R, Derdour A, Bengueddach A, Mongin F (2015c) Catalytic behavior of surfactant-containing-MCM-41 mesoporous materials for cycloaddition of 4-nitrophenyl azide. Appl Catal A 489:131–139. doi:10.1016/j.apcata.2014.10.022
Boukoussa B, Hamacha R, Morsli A, Bengueddach A (2017) Adsorption of yellow dye on calcined or uncalcined Al-MCM-41 mesoporous materials. Arabian J Chem 10:S2160–S2169. doi:10.1016/j.arabjc.2013.07.049
Brahmi L, Ali-Dahmane T, Hamacha R, Hacini S (2016) Catalytic performance of Al-MCM-41 catalyst for the allylation of aromatic aldehydes with allyltrimethylsilane: comparison with TiCl4 as Lewis acid. J Mol Catal A Chem 423:31–40. doi:10.1016/j.molcata.2016.06.004
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319. doi:10.1021/ja01269a023
Burgoyne RA, Meijboom R (2013) Knoevenagel condensation reactions catalysed by metal-organic frameworks. Catal Lett 143:563–571. doi:10.1007/s10562-013-0995-5
Chikh K, Boukoussa B, Bouhadjar L, Bencheikh M, Hamacha R, Meghabar R, Belbachir M, Bengueddach A (2015) Polymerization of pyrrole with 4-hydroxybenzaldehyde over Al-MCM-41 mesoporous aluminosilicate materials. Res Chem Intermed 41:6485–6496. doi:10.1007/s11164-014-1755-x
Davis ME (2002) Ordered porous materials for emerging applications. Nature 417:813–821. doi:10.1038/nature00785
Deepak BN, Rana S, Parida K, Bhanage BM (2014) Amine functionalized MCM-41 as a green, efficient, and heterogeneous catalyst for the regioselective synthesis of 5-aryl-2-oxazolidinones, from CO2 and aziridines. Appl Catal A 469:340–349
Gholami Z, Abdullah AZ, Lee KT (2014) Heterogeneously catalyzed etherification of glycerol to diglycerol over calcium–lanthanum oxide supported on MCM-41: a heterogeneous basic catalyst. Appl Catal A 479:76–86. doi:10.1016/j.apcata.2014.04.024
Guangcai Z, Tong Z, Xiongfu Z, King Lun Y (2015) Continuous flow ZIF-8/NaA composite membrane microreactor for efficient Knoevenagel condensation. Catal Commun 68:93–96. doi:10.1016/j.catcom.2015.05.008
Huang J, Ding S, Xiao W, Peng Y, Deng S, Zhang N (2015) 3-Aminopropyl-triethoxysilane functionalized graphene oxide: a highly efficient and recyclable catalyst for Knoevenagel condensation. Catal Lett 145:1000–1007. doi:10.1007/s10562-014-1461-8
Izquierdo-Barba I, Sánchez-Salcedo S, Colilla M, Feito MJ, Ramírez-Santillán C, Portolés MT, Vallet-Regí M (2011) Inhibition of bacterial adhesion on biocompatible zwitterionic SBA-15 mesoporous materials. Acta Biomater 7:2977–2985. doi:10.1016/j.actbio.2011.03.005
Jlalia I, Gallier F, Brodie-Linder N, Uziel J, Augé J, Lubin-Germain N (2014) Copper(II) SBA-15: a reusable catalyst for azide–alkyne cycloaddition. J Mol Catal A: Chem 393:56–61. doi:10.1016/j.molcata.2014.06.003
Jun X, Lang C, Au C-T, Shuang-Feng Y (2015) Synthesis of KOH/SnO2 solid superbases for catalytic Knoevenagel condensation. Catal Commun 66:30–33. doi:10.1016/j.catcom.2015.03.008
Keita I, Keita M, Takayuki T, Masato M (2011) ca-containing mesoporous silica as a solid base catalyst for the Knoevenagel condensation reaction. Catal Lett 141:877–881. doi:10.1007/s1056-011-0613-3
Kibou Z, Cheikh N, Villemin D, Choukchou-Braham N, Mostefa-Kara B, Benabdallah M (2011) A simple and efficient procedure for a 2-pyridones synthesis under solvent-free conditions. Int J Org Chem 1:242–249. doi:10.4236/ijoc.2011.14035
Knoevenagel E (1898) Condensationen zwischen Malonester und Aldehyden unter dem Einfluss von Ammoniak und organischen Aminen. Berichte 31:2585–2596. doi:10.1002/cber.18980310307
Koller H, Lobo RF, Burkett SL, Davis ME (1995) SiO–…HOSi hydrogen bonds in as-synthesized high-silica zeolites. J Phys Chem 99:12588–12596. doi:10.1021/j100033a036
Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS (1992) Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 359:710–712. doi:10.1038/359710a0
Kubota Y, Ikeya H, Sugi Y, Yamada T, Tatsumi T (2006) Organic–inorganic hybrid catalysts based on ordered porous structures for Michael reaction. J Mol Catal A: Chem 249:181–190. doi:10.1016/j.molcata.2006.01.015
Lixin X, Ye Z, Cui Q, Zhiyong G, Mercier L (2011) Surface-initiated catalytic ethylene polymerization within nano-channels of ordered mesoporous silicas for synthesis of hybrid silica composites containing covalently tethered polyethylene. Polymer 52:5961–5974. doi:10.1016/j.polymer.2011.11.011
Lu L, Kejing Q, Xu J, Fusheng L, Shiwei L, Yu S, Congxia X, Xiaoping G (2014) Preparation of basic mesoporous molecular sieves K2O/Mg-MCM-41 and its catalytic performance on the cracking of soybean oils. J Anal Appl Pyrol 110:313–317. doi:10.1016/j.jaap.2014.09.019
Martins L, Bonagamba TJ, de Azevedo ER, Bargiela P, Di Cardoso (2006) Surfactant containing Si-MCM-41: an efficient basic catalyst for the Knoevenagel condensation. Appl Catal A 312:77–85. doi:10.1016/j.apcata.2006.06.035
Martins L, Hölderich W, Hammer P, Cardoso D (2010) Preparation of different basic Si–MCM-41 catalysts and application in the Knoevenagel and Claisen-Schmidt condensation reactions. J Catal 271:220–227. doi:10.1016/j.jcat.2010.01.015
Morsli A, Benhamou A, Basly JP, Baudu M, Derriche Z (2015) Mesoporous silicas: improving the adsorption efficiency of phenolic compounds by the removal of amino group from functionalized silicas. RSC Adv 5:41631–41638. doi:10.1039/C5RA03066H
Oliveira AC, Martins L, Cardoso D (2009) Basic catalytic properties of as-synthesized molecular sieves. Microporous Mesoporous Mater 120:206–213. doi:10.1016/j.micromeso.2008.10.033
Ouargli-Saker R, Bouazizi N, Boukoussa B, Barrimo D, Paola-Nunes-Beltrao A, Azzouz A (2017) Metal-loaded SBA-16-like silica—correlation between basicity and affinity towards hydrogen. Appl Surf Sci 411:476–486. doi:10.1016/j.apsusc.2017.03.165
Parangi TF, Patel RM, Chudasama UV (2014) Synthesis and characterization of mesoporous Si-MCM-41 materials and their application as solid acid catalysts in some esterification reactions. Bull Mater Sci 37:609–615. doi:10.1007/s12034-014-0709-7
Parida KM, Dharitri R (2009) Amine functionalized MCM-41: an active and reusable catalyst for Knoevenagel condensation reaction. J Mol Catal A Chem 310:93–100. doi:10.1016/j.molcata.2009.06.001
Pauly TR, Liu Y, Pinnavaia TJ, Billinge SJL, Rieker TP (1999) Textural mesoporosity and the catalytic activity of mesoporous molecular sieves with wormhole framework structures. J Am Chem Soc 121:8835–8842. doi:10.1021/ja991400t
Pirouzmand M, Nikzad-kojanag B, Seyed-Rasulzade SK (2015) Surfactant containing Ca/MCM-41 as a highly active, green and reusable catalyst for the trans esterification of canola oil. Catal Commun 69:196–201. doi:10.1016/j.catcom.2015.06.021
Prajpati D, Lekhok KC, Sandhu JS, Ghosh AC (1996) Lithium bromide as a new catalyst for carbon–carbon bond formation in the solid state. J Chem Soc Pekin Trans 1:959. doi:10.1039/P19960000959
Ranucci CR, Colpini LMS, Monteiro MR, Kothe V, Gasparrini LJ, Alves HJ (2015) Preparation, characterization and stability of KF/Si-MCM-41 basic catalysts for application in soybean oil transesterification with methanol. J Environ Chem Eng 3:703–707. doi:10.1016/j.jmst.2016.08.025
Rao PS, Venkataratnam RV (1991) Zinc chloride as a new catalyst for Knoevenagel condensation. Tetrahedron Lett 32:5821–5822. doi:10.1016/S0040-4039(00)93564-0
Sekkiou H, Boukoussa B, Ghezini R, Khenchoul Z, Ouali A, Hamacha R, Bengueddach A (2016) Enhanced hydrogen storage capacity of copper containing mesoporous silicas prepared using different methods. Mater Res Express 3:085501. doi:10.1088/2053-1591/3/8/085501
Semsarzadeh MA, Amiri S, Azadeh M (2012) Controlled radical polymerization of vinyl acetate in presence of mesoporous silica supported TiCl heterogeneous catalyst. Bull Mater Sci 35:867–874. doi:10.1007/s12034-012-0361-z
Srivastava R, Srinivas D, Ratnasamy P (2006) Syntheses of polycarbonate and polyurethane precursors utilizing CO2 over highly efficient, solid as-synthesized MCM-41 catalyst. Tetrahedron Lett 47:4213–4217. doi:10.1016/j.tetlet.2006.04.057
Sunghwan P, Joona B, Jungkyu C, Sang Hyup L, Jung-Hyun L, Jong Suk L (2014) 3-Dimensionally disordered mesoporous silica (DMS)-containing mixed matrix membranes for CO2 and non-CO2 greenhouse gas separations. Sep Purif Technol 136:286–295. doi:10.1016/j.seppur.2014.09.016
Talha Z, Bachir C, Ziri S, Bellahouel S, Bengueddach A, Villièras F, Pelletier M, Weidler PG, Hamacha R (2017) Al-rich ordered mesoporous silica SBA-15 materials: synthesis. Surf Charact Acid Prop. doi:10.1007/s10562-017-2103-8
Terrab I, Ouargli R, Boukoussa B, Ghomari K, Hamacha R, Roy R, Azzouz A, Bengueddach A (2017) Assessment of the intrinsic interactions of mesoporous silica with carbon dioxide. Res Chem Intermed 43:3775–3786. doi:10.1007/s11164-016-2846-7
Venkatesan C, Chidambaram M, Singh AP (2005) 3-Aminopropyltriethoxysilyl functionalized Na-Al-MCM-41 solid base catalyst for selective preparation of 2-phenylpropionitrile from phenylacetonitrile. Appl Catal A 292:344–353. doi:10.1016/j.apcata.2005.06.013
Wach A, Drozdek M, Dudek B, Szneler E, Kuśtrowski P (2015) Control of amine functionality distribution in polyvinylamine/SBA-15 hybrid catalysts for Knoevenagel condensation. Catal Commun 64:52–57. doi:10.1016/j.catcom.2015.02.002
Wu S, Song K, Guan J, Kan Q (2011) Synthesis and characterization of super-microporous material with enhanced hydrothermal stability. Bull Mater Sci 34:979–983. doi:10.1007/s12034-011-0225-y
Zhu F, Sun Xiaojun, Lou Fengwen, An Litao, Zhao Pusu (2015) Facile one-pot synthesis of amine-functionalized mesoporous silica nanospheres for water-medium Knoevenagel reaction under microwave irradiation. Catal Lett 145:1072–1079. doi:10.1007/s10562-015-1484-9
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Boukoussa, B., Kibou, Z., Abid, Z. et al. Key factor affecting the basicity of mesoporous silicas MCM-41: effect of surfactant extraction time and Si/Al ratio. Chem. Pap. 72, 289–299 (2018). https://doi.org/10.1007/s11696-017-0279-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-017-0279-4