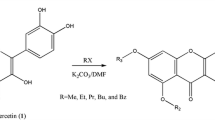
Abstract
A set of four C-8-aminomethyl derivatives of quercetin has been synthesized by Mannich reaction. The synthesis was carried out using a simple procedure to give target compounds as hydrochlorides. Study of oxidative hemolysis on mice erythrocytes showed that derivatives with morpholinomethyl or thiomorpholinomethyl groups favorably differ from the original quercetin by the ability to protect cells from acute oxidative stress.
Graphical abstract


Similar content being viewed by others
References
Acker CI, Brandão R, Rosário AR, Nogueira CW (2009) Antioxidant effect of alkynylselenoalcohol compounds on liver and brain of rats in vitro. Environ Toxicol Pharmacol 28:280–287. doi:10.1016/j.etap.2009.05.002
Al-Ghorbani M, Bushra BA, Zabiulla MSV, Khanum S (2015) Piperazine and morpholine: synthetic preview and pharmaceutical applications. Res J Pharm Technol 8:611–628. doi:10.5958/0974-360X.2015.00100.6
Arora A, Byrem TM, Nair MG, Strasburg GM (2000) Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Arch Biochem Biophys 373:102–109. doi:10.1006/abbi.1999.1525
Asakawa T, Matsushita S (1980) Coloring conditions of thiobarbituric acid test for detecting lipid hydroperoxides. Lipids 15:137–140. doi:10.1007/BF02540959
Bellé NAV, Dalmolin GD, Fonini G, Rubin MA, Rocha JBT (2004) Polyamine reduces lipid peroxidation induced by different pro-oxidant agents. Brain Res 1008:245–251. doi:10.1016/j.brainres.2004.02.036
Buravlev EV, Chukicheva IY, Suponitsky KY, Vikharev YB, Grishko VV, Kutchin AV (2011) Synthesis and biological evaluation of enantioenriched phenols having an isobornyl substituent. Lett Org Chem 8:301–307. doi:10.2174/157017811795685054
Buravlev EV, Shevchenko OG, Kutchin AV (2015) Synthesis and membrane-protective activity of novel derivatives of α-mangostin at the C-4 position. Bioorg Med Chem Lett 25:826–829. doi:10.1016/j.bmcl.2014.12.075
Chawla R, Arora R, Kumar R, Sharma A, Prasad J, Singh S, Sagar R, Chaudhary P, Shukla S, Kaur G, Sharma RK, Puri SC, Dhar KL, Handa G, Gupta VK, Qazi GN (2005) Antioxidant activity of fractionated extracts of rhizomes of high-altitude Podophyllumhexandrum: role in radiation protection. Mol Cell Biochem 273:193–208. doi:10.1007/s11010-005-0821-5
Chen Y, Deuster P (2009) Comparison of quercetin and dihydroquercetin: antioxidant-independent actions on erythrocyte and platelet membrane. Chem Biol Interact 182:7–12. doi:10.1016/j.cbi.2009.06.007
Chen L, Hu TS, Zhu J, Wu H, Yao ZJ (2006) Application of a regioselective Mannich reaction on naringenin and its use in fluorescent labeling. Synlett 8:1225–1229. doi:10.1055/s-2006-941564
Chrysselis MC, Rekka EA, Kourounakis PN (2000) Hypocholesterolemic and hypolipidemic activity of some novel morpholine derivatives with antioxidant activity. J Med Chem 43:609–612. doi:10.1021/jm991039l
Cross HJ, Tilby M, Chipman JK, Ferry DR, Gescher A (1996) Effect of quercetin on the genotoxic potential of cisplatin. Int J Cancer 66:404–408. doi:10.1002/(SICI)1097-0215(19960503)66:3<404::AID-IJC23>3.0.CO;2-9
Donato L, Chiappetta G, Drioli E (2011) Surface functionalization of PVDF membrane with a naringin-imprinted polymer layer using photo-polymerization method. Sep Sci Technol 46:1555. doi:10.1080/01496395.2011.575429
Erlund I (2004) Review of the flavonoids quercetin, hesperetin and naringenin. Dietary sources, bioactivities, and epidemiology. Nutr Res 24:851–874. doi:10.1016/j.nutres.2004.07.005
Fatokun AA, Tome M, Smith RA, Darlington LG, Stone TW (2015) Protection by the flavonoids quercetin and luteolin against peroxide- or menadione-induced oxidative stress in MC3T3-E1 osteoblast cells. Nat Prod Res 29:1127–1132. doi:10.1080/14786419.2014.980252
Gul HI, Sahin F, Ful M, Ozturk S, Yerdelen KO (2005) Evaluation of antimicrobial activities of several Mannich bases and their derivatives. Arch Pharm Chem Life Sci 338:335–338. doi:10.1002/ardp.200400962
Hapner CD, Deuster P, Chen Y (2010) Inhibition of oxidative hemolysis by quercetin, but not other antioxidants. Chem Biol Interact 186:275–279. doi:10.1016/j.cbi.2010.05.010
Harwood M, Danielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, Lines TC (2007) A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol 45:2179–2205. doi:10.1016/j.fct.2007.05.015
Heim KE, Tagliaferro AR, Bobilya DJ (2002) Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem 13:572–584. doi:10.1016/S0955-2863(02)00208-5
Helgren TR, Sciotti RJ, Lee P, Duffy S, Avery VM, Igbinoba O, Akoto M, Hagen TJ (2015) The synthesis, antimalarial activity and CoMFA analysis of novel aminoalkylated quercetin analogs. Bioorg Med Chem Lett 25:327–332. doi:10.1016/j.bmcl.2014.11.039
Ilkei V, Spaits A, Prechl A, Müller J, Könczöl Á, Lévai S, Riethmüller E, Szigetvári Á, Béni Z, Dékány M, Martins A, Hunyadi A, Antus S, Szántay C Jr, Balogh GT, Kalaus G, Bölcskei H, Hazai L (2017) C8-selective biomimetic transformation of 5,7-dihydroxylated flavonoids by an acid-catalysed phenolic Mannich reaction: synthesis of flavonoid alkaloids with quercetin and (–)-epicatechin skeletons. Tetrahedron 73:1503–1510. doi:10.1016/j.tet.2017.01.068
Joshi D, Field J, Murphy J, Abdelrahim M, Schonherr H, Sparrow JR, Ellestad G, Nakanishi K, Zask A (2013) Synthesis of antioxidants for prevention of age-related macular degeneration. J Nat Prod 76:450–454. doi:10.1021/np300769c
Kao TH, Huang SC, Inbaraj BS, Chen BH (2008) Determination of flavonoids and saponins in Gynostemma pentaphyllum (Thunb.) Makino by liquid chromatography–mass spectrometry. Anal Chim Acta 626:200–211. doi:10.1016/j.aca.2008.07.049
Kim JS (2013) Preliminary evaluation for comparative antioxidant activity in the water and ethanol extracts of dried citrus fruit (Citrus unshiu) peel using chemical and biochemical in vitro assays. Food Nutr Sci 4:177–188. doi:10.4236/fns.2013.42025
Kucukoglu K, Gul M, Atalay M, Mete E, Kazaz C, Hanninen O, Gul HI (2011) Synthesis of some Mannich bases with dimethylamine and their hydrazones and evaluation of their cytotoxicity against Jurkat cells. Arzneimittelforschung 61:366–371. doi:10.1055/s-0031-1296212
Lim SN, Cheung PCK, Ooi VEC, Ang PO (2002) Evaluation of antioxidative activity of extracts from a brown seaweed, Sargassum siliquastrum. J Agric Food Chem 50:3862–3866. doi:10.1021/jf020096b
López-Revuelta A, Sánchez-Gallego JI, Hernández-Hernández A, Sánchez-Yagüe J, Llanillo M (2006) Membrane cholesterol contents influence the protective effects of quercetin and rutin in erythrocytes damaged by oxidative stress. Chem Biol Interact 161:79–91. doi:10.1016/j.cbi.2006.03.004
Markham KR, Ternai B, Stanley R, Geiger H, Mabry TJ (1978) Carbon-13 NMR studies of flavonoids–III. Naturally occurring flavonoid glycosides and their acylated derivatives. Tetrahedron 34:1389–1397. doi:10.1016/0040-4020(78)88336-7
Middleton E, Kandaswami C, Theoharides TC (2000) The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev 52:673–751
Nagababu E, Rifkind JM (1998) Formation of fluorescent heme degradation products during the oxidation of hemoglobin by hydrogen peroxide. Biochem Biophys Res Commun 247:592–596. doi:10.1006/bbrc.1998.8846
Nagababu E, Fabry ME, Nagel RL, Rifkind JM (2008) Heme degradation and oxidative stress in murine models for hemoglobinopathies: thalassemia, sickle cell disease and hemoglobin C disease. Blood Cells Mol Dis 41:60–66. doi:10.1016/j.bcmd.2007.12.003
Nagababu E, Mohanty JG, Bhamidipaty S, Ostera GR, Rifkind JM (2010) Role of the membrane in the formation of heme degradation products in red blood cells. Life Sci 86:133–138. doi:10.1016/j.lfs.2009.11.015
Naim MJ, Alam O, Alam MJ, Alam P, Shrivastava N (2015) A review on pharmacological profile of morpholine derivatives. Int J Pharmacol Pharm Sci 3(1):40–51
Nasser II, Algieri C, Garofalo A, Drioli E, Ahmed C, Donato L (2016) Hybrid imprinted membranes for selective recognition of quercetin. Sep Purif Technol 163:331–340. doi:10.1016/j.seppur.2016.03.015
Roman G (2015) Mannich bases in medicinal chemistry and drug design. Eur J Med Chem 89:743–816. doi:10.1016/j.ejmech.2014.10.076
Sevgi K, Tepe B, Sarikurkcu C (2015) Antioxidant and DNA damage protection potentials of selected phenolic acids. Food Chem Toxicol 77:12–21. doi:10.1016/j.fct.2014.12.006
Stefanello ST, Prestes AS, Ogunmoyole T, Salman SM, Schwab RS, Brender CR, Dornelles L, Rocha JBT, Soares FAA (2013) Evaluation of in vitro antioxidant effect of new mono and diselenides. Toxicol In Vitro 27:1433–1439. doi:10.1016/j.tiv.2013.03.001
Suwalsky M, Vargas P, Avello M, Villena F, Sotomayor CP (2008) Human erythrocytes are affected in vitro by flavonoids of Aristotelia chilensis (Maqui) leaves. Int J Pharm 363:85–90. doi:10.1016/j.ijpharm.2008.07.005
Takebayashi J, Chen J, Tai A (2010) Chapter 20, A method for evaluation of antioxidant activity based on inhibition of free radical-induced erythrocyte hemolysis. In: Armstrong A (ed) Advanced protocols in oxidative stress II (methods in molecular biology). Humana Press, New York. doi:10.1007/978-1-60761-411-1_20
Tramontini M, Angiolini L (1994) Mannich bases: chemistry and uses. CRC Press, Boca Raton
van den Berg JJM, Op den Kamp JAF, Lubin BH, Roelofsen B, Kuypers FA (1992) Kinetics and site specificity of hydroperoxide-induced oxidative damage in red blood cells. Free Radic Biol Med 12:487–498. doi:10.1016/0891-5849(92)90102-M
Veverka M, Gallovic J, Svajdlenka E, Veverkova E, Pronayova N, Milackova I, Stefek M (2013) Novel quercetin derivatives: synthesis and screening for anti-oxidant activity and aldose reductase inhibition. Chem Pap 67:76–83. doi:10.2478/s11696-012-0240-5
Wu CR, Lin WH, Hseu YC, Lien JC, Lin YT, Kuo TP, Ching H (2011) Evaluation of the antioxidant activity of five endemic Ligustrum species leaves from Taiwan flora in vitro. Food Chem 127:564–571. doi:10.1016/j.foodchem.2011.01.041
Zhan W, Lin S, Chen J, Dong X, Chu J, Du W (2015) Design, synthesis, biological evaluation, and molecular docking of novel benzopyran and phenylpyrazole derivatives as Akt inhibitors. Chem Biol Drug Des 85:770–779. doi:10.1111/cbdd.12489
Zhang S, Ma J, Bao Y, Yang P, Zou L, Li K, Sun X (2008) Nitrogen-containing flavonoid analogues as CDK1/cyclin B inhibitors: synthesis, SAR analysis, and biological activity. Bioorg Med Chem 16:7127–7132. doi:10.1016/j.bmc.2008.06.055
Zhang M, Swarts SG, Yin L, Liu C, Tian Y, Cao Y, Swarts M, Yang S, Zhang SB, Zhang K, Ju S, Olek DJ Jr, Schwartz L, Keng PC, Howell R, Zhang L, Okunieff P (2011) Antioxidant properties of quercetin. Adv Exp Med Biol 701:283–289. doi:10.1007/978-1-4419-7756-4_38
Acknowledgements
The spectral data were obtained using the equipment of the Center of Collective Usage (CCU) ‘Chemistry’, Institute of Chemistry, Komi Scientific Center, Ural Branch of the RAS. The study of the biological activity of the compounds was done using the equipment of the CCU ‘Molecular Biology’, Institute of Biology, Komi Scientific Center, Ural Branch of the RAS. The mice of the scientific collection of experimental animals of Institute of Biology, Komi Scientific Center, Ural Branch of the RAS were used (http://www.ckp-rf.ru/usu/471933/). This study was funded by the Russian Foundation for Basic Research (Grant no. 16-53-00171).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Buravlev, E.V., Shevchenko, O.G., Chukicheva, I.Y. et al. Synthesis and membrane-protective properties of aminomethyl derivatives of quercetin at the C-8 position. Chem. Pap. 72, 201–208 (2018). https://doi.org/10.1007/s11696-017-0272-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-017-0272-y