Abstract
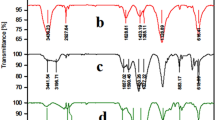
In the present work, nano-ceramic tile wastes were used as accessible, nontoxic and inexpensive support materials for the immobilization of phosphomolybdic acid (H3PMo12O40) in 1–20 wt% by an impregnation method. The novel and active heterogeneous solid acid nanocatalyst, so-called nano-ceramic tile waste, supported phosphomolybdic acid (n-CTW/PMA) well characterized by the aid of FT-IR, XRD, FE-SEM, EDX and TGA analyses. The catalytic activity of the as-prepared catalyst was probed in the chemoselective oxidation of sulfides to sulfoxides using 30% H2O2 as green oxidant at room temperature under solvent-free conditions. Optimization of the reaction conditions was accomplished by the aid of central composite design (CCD) as one of the most applicable response surface methodologies. The results showed that the catalyst with 11 wt% PMA loading led to high conversion rates and yields (97%). Besides, the noticeable advantages of this new catalyst were as follows: highly active and selective, low-toxic and -cost, available and stable, recoverable and reusable for several times.
Graphical abstract












Similar content being viewed by others
References
Anastas PT, Kirchhoff MM (2002) Origins, current status, and future challenges of green chemistry. Acc Chem Res 35:686–694. doi:10.1021/ar010065m
Atia H, Armbruster U, Martin A (2008) Dehydration of glycerol in gas phase using heteropolyacid catalysts as active compounds. J Catal 258:71–82. doi:10.1016/j.jcat.2008.05.027
Azar ARJ, Safaei E, Mohebbi S (2015) A novel Schiff base of Mn(III) complex supported on magnetic cobalt nanoparticles as a highly efficient retrievable heterogeneous catalyst in oxidation of alcohols and sulfides compounds. Mater Res Bull 70:753–776. doi:10.1016/j.materresbull.2015.05.016
Barbaro P, Liguori F (2008) Ion exchange resins: catalyst recovery and recycle. Chem Rev 109:515–529. doi:10.1021/cr800404j
Benaglia M, Puglisi A, Cozzi F (2003) Polymer-supported organic catalysts. Chem Rev 103:3401–3430. doi:10.1021/cr010440o
Bhat AH, Khalil HPSA, Mishra RK, Datt M, Banthia AK (2011) Development and material properties of chitosan and phosphomolybdic acid-based composites. J Compos Mater 45:39–49. doi:10.1177/0021998310371552
Block E (1978) Reactions of organosulfur compounds: organic chemistry: a series of monographs. Academic Press, Cambridge
Cao J, He N, Li C, Dong J, Xu Q (1998) Fe-containing mesoporous molecular sieves as benzylation catalysts. Stud Surf Sci Catal 117:461–467. doi:10.1016/s0167-2991(98)81025-2
Carrasco CJ, Montilla F, Bobadilla L, Ivanova S, Odriozola JA, Galindo A (2015) Oxodiperoxomolybdenum complex immobilized onto ionic liquid modified SBA-15 as an effective catalysis for sulfide oxidation to sulfoxides using hydrogen peroxide. Catal Today 255:102–108. doi:10.1016/j.cattod.2014.10.053
Clark JH, Cullen SR, Barlow SJ, Bastock TW (1994) Keynote article. Environmentally friendly chemistry using supported reagent catalysts: structure–property relationships for clayzic. J Chem Soc, Perkin Trans 2:1117–1130. doi:10.1039/P29940001117
Cundy CS, Cox PA (2003) The hydrothermal synthesis of zeolites: history and development from the earliest days to the present time. Chem Rev 103:663–702. doi:10.1021/cr020060i
Das B, Chowdhury N (2007) Amberlyst-15: an efficient reusable heterogeneous catalyst for aza-Michael reactions under solvent-free conditions. J Mol Catal A: Chem 263:212–215. doi:10.1016/j.molcata.2006.08.082
Dasgupta S, Torok B (2008) Environmentally benign contemporary Friedel-Crafts chemistry by solid acids. Curr Org Synth 5:321–342. doi:10.2174/157017908786241572
Davis ME, Lobo RF (1992) Zeolite and molecular sieve synthesis. Chem Mater 4:756–768
El-Wahab MMMA, Said AA (2005) Phosphomolybdic acid supported on silica gel and promoted with alkali metal ions as catalysts for the esterification of acetic acid by ethanol. J Mol Catal A: Chem 240:109–118. doi:10.1016/j.molcata.2005.06.038
Figueras F, Kantam ML, Choudary BM (2006) Solid base catalysts in organic synthesis. Curr Org Chem 10:1627–1637. doi:10.2174/138527206778249658
García-Gutiérrez JL, Fuentes GA, Hernández-Terán ME, Murrieta F, Navarrete J, Jiménez-Cruz F (2006) Ultra-deep oxidative desulfurization of diesel fuel with H2O2 catalyzed under mild conditions by polymolybdates supported on Al2O3. Appl Catal A 305:15–20. doi:10.1016/j.apcata.2006.01.027
García-Gutiérrez JL, Fuentes GA, Hernández-Terán ME, Garcia P, Murrieta-Guevara F, Jiménez-Cruz F (2008) Ultra-deep oxidative desulfurization of diesel fuel by the Mo/Al2O3–H2O2 system: the effect of system parameters on catalytic activity. Appl Catal A 334:366–373. doi:10.1016/j.apcata.2007.10.024
Ghanbari-Siahkali A, Philippou A, Dwyer J, Anderson MW (2000) The acidity and catalytic activity of heteropoly acid on MCM-41 investigated by MAS NMR, FTIR and catalytic tests. Appl Catal A 192:57–69. doi:10.1016/S0926-860X(99)00333-6
Ghorbani-Choghamarani A, Ghasemi B, Safari Z, Azadi G (2015) Schiff base complex coated Fe 3 O 4 nanoparticles: a highly reusable nanocatalyst for the selective oxidation of sulfides and oxidative coupling of thiols. Catal Commun 60:70–75. doi:10.1016/j.catcom.2014.11.007
Goesmann H, Feldmann C (2010) Nanoparticulate functional materials. Angew Chem Int Ed 49:1362–1395. doi:10.1002/anie.200903053
Gruttadauria M, Giacalone F, Noto R (2008) Supported proline and proline-derivatives as recyclable organocatalysts. Chem Soc Rev 37:1666–1688. doi:10.1039/B800704G
Hara M, Yoshida T, Takagaki A, Takata T, Kondo JN, Hayashi S, Domen K (2004) A carbon material as a strong protonic acid. Angew Chem Int Ed 43:2955–2958. doi:10.1002/anie.200453947
He NY, Woo CS, Kim HG, Lee HI (2005) Catalytic formation of acetic anhydride over tungstophosphoric acid modified SBA-15 mesoporous materials. Appl Catal A 281:167–178. doi:10.1016/j.apcata.2004.11.026
Hosseini MM, Kolvari E, Koukabi N, Ziyaei M, Zolfigol MA (2016) Zirconia sulfuric acid: an efficient heterogeneous catalyst for the one-pot synthesis of 3, 4-dihydropyrimidinones under solvent-free conditions. Catal Lett 146:1040–1049. doi:10.1007/s10562-016-1723-8
Izumi Y, Urabe K, Onaka M (1992) Zeolite, clay, and heteropoly acid in organic reactions ed^eds: VCH
Kalbasi RJ, Ghiaci M, Massah AR (2009) Highly selective vapor phase nitration of toluene to 4-nitro toluene using modified and unmodified H 3PO 4/ZSM-5. Appl Catal A 353:1–8. doi:10.1016/j.apcata.2008.10.013
Keggin JF (1933) Structure of the molecule of 12-phosphotungstic acid. Nature 131:908–909. doi:10.1038/131908b0
Kiss AA, Dimian AC, Rothenberg G (2006) Solid acid catalysts for biodiesel production—towards sustainable energy. Adv Synth Catal 348:75–81. doi:10.1002/adsc.200505160
Kolvari E, Zolfagharinia S (2016) A waste to wealth approach through utilization of nano-ceramic tile waste as an accessible and inexpensive solid support to produce a heterogeneous solid acid nanocatalyst: to kill three birds with one stone. RSC Adv 6:93963–93974. doi:10.1039/C6RA11923A
Kolvari E, Koukabi N, Armandpour O (2014) A simple and efficient synthesis of 3,4-dihydropyrimidin-2-(1H)-ones via Biginelli reaction catalyzed by nanomagnetic-supported sulfonic acid. Tetrahedron 70:1383–1386. doi:10.1016/j.tet.2013.10.085
Kolvari E, Koukabi N, Hosseini MM (2015a) Perlite: a cheap natural support for immobilization of sulfonic acid as a heterogeneous solid acid catalyst for the heterocyclic multicomponent reaction. J Mol Catal A: Chem 397:68–75. doi:10.1016/j.molcata.2014.10.026
Kolvari E, Koukabi N, Hosseini MM, Khandani Z (2015b) Perlite: an inexpensive natural support for heterogenization of HBF4. RSC Adv 5:36828–36836. doi:10.1039/C5RA03229F
Kolvari E, Koukabi N, Hosseini MM, Vahidian M, Ghobadi E (2016a) Nano-ZrO2 sulfuric acid: a heterogeneous solid acid nano catalyst for Biginelli reaction under solvent free conditions. RSC Adv 6:7419–7425. doi:10.1039/C5RA19350H
Kolvari E, Zolfagharinia S, Koukabi N (2016b) A unique opportunity for the utilization of glass wastes as a resource for catalytic applications: toward a cleaner environment. RSC Adv 6:113844–113858. doi:10.1039/C6RA22791K
Kon Y, Yokoi T, Yoshioka M, Tanaka S, Uesaka Y, Mochizuki T, Sato K, Tatsumi T (2014) Selective hydrogen peroxide oxidation of sulfides to sulfoxides or sulfones with MWW-type titanosilicate zeolite catalyst under organic solvent-free conditions. Tetrahedron 70:7584–7592. doi:10.1016/j.tet.2014.07.091
Koukabi N, Kolvari E, Khazaei A, Zolfigol MA, Shaghasemi BS, Khavasi HR (2011) Hantzsch reaction on free nano-Fe2O3 catalyst: excellent reactivity combined with facile catalyst recovery and recyclability. Chem Commun 47:9230–9232. doi:10.1039/C1CC12693H
Koukabi N, Kolvari E, Zolfigol MA, Khazaei A, Shaghasemi BS, Fasahatib B (2012) A magnetic particle-supported sulfonic acid catalyst: tuning catalytic activity between homogeneous and heterogeneous catalysis. Adv Synth Catal 354:2001–2008. doi:10.1002/adsc.201100352
Langpape M, Millet JMM, Ozkan US, Boudeulle M (1999) Study of cesium or cesium-transition metal-substituted Keggin-type phosphomolybdic acid as isobutane oxidation catalysts: I. Structural characterization. J Catal 181:80–90. doi:10.1006/jcat.1998.2217
Lee I, Albiter MA, Zhang Q, Ge J, Yin Y, Zaera F (2011) New nanostructured heterogeneous catalysts with increased selectivity and stability. Phys Chem Chem Phys 13:2449–2456. doi:10.1039/C0CP01688H
Liu J, Yang Q, Kapoor MP, Setoyama N, Inagaki S, Yang J, Zhang L (2005) Structural relation properties of hydrothermally stable functionalized mesoporous organosilicas and catalysis. J Phys Chem B 109:12250–12256. doi:10.1021/jp0509109
Liu F, Meng X, Zhang Y, Ren L, Nawaz F, Xiao F-S (2010) Efficient and stable solid acid catalysts synthesized from sulfonation of swelling mesoporous polydivinylbenzenes. J Catal 271:52–58. doi:10.1016/j.jcat.2010.02.003
Liu F, Kong W, Qi C, Zhu L, Xiao F-S (2012) Design and synthesis of mesoporous polymer-based solid acid catalysts with excellent hydrophobicity and extraordinary catalytic activity. ACS Catal 2:565–572. doi:10.1021/cs200613p
Lv Z, Sun Q, Meng X, Xiao F-S (2013) Superhydrophilic mesoporous sulfonated melamine–formaldehyde resin supported palladium nanoparticles as an efficient catalyst for biofuel upgrade. J Mater Chem A 1:8630–8635. doi:10.1039/C3TA10916J
Mata EG (1996) Phosphorus sulfur silicon, 117: 231. Taylor & Francis Online] [Web of Science®]. doi:10.1080/10426509608038790
Mbaraka IK, Radu DR, Lin VSY, Shanks BH (2003) Organosulfonic acid-functionalized mesoporous silicas for the esterification of fatty acid. J Catal 219:329–336. doi:10.1016/S0021-9517(03)00193-3
Melero JA, Iglesias J, Morales G (2009) Heterogeneous acid catalysts for biodiesel production: current status and future challenges. Green Chem 11:1285–1308. doi:10.1039/B902086A
Misono M, Mizuno N, Katamura K, Kasai A, Konishi Y, Sakata K, Okuhara T, Yoneda Y (1982) Catalysis by heteropoly compounds. III. The structure and properties of 12-heteropolyacids of molybdenum and tungsten (H3PMo12 − x Wx O40) and their salts pertinent to heterogeneous catalysis. Bull Chem Soc Jpn 55:400–406. doi:10.1246/bcsj.55.400
Mizuno N, Misono M (1998) Heterogeneous catalysis. Chem Rev 98:199–218. doi:10.1021/cr960401q
Nakajima K, Okamura M, Kondo JN, Domen K, Tatsumi T, Hayashi S, Hara M (2008) Amorphous carbon bearing sulfonic acid groups in mesoporous silica as a selective catalyst. Chem Mater 21:186–193. doi:10.1021/cm801441c
Nikoorazm M, Ghorbani-Choghamarani A, Mahdavi H, Esmaeili SM (2015) Efficient oxidative coupling of thiols and oxidation of sulfides using UHP in the presence of Ni or Cd salen complexes immobilized on MCM-41 mesoporous as novel and recoverable nanocatalysts. Micropor Mesopor Mat 211:174–181. doi:10.1016/j.micromeso.2015.03.011
Noji M, Konno Y, Ishii K (2007) Metal triflate-catalyzed cationic benzylation and allylation of 1, 3-dicarbonyl compounds. J Org Chem 72:5161–5167. doi:10.1021/jo0705216
Okamura M, Takagaki A, Toda M, Kondo JN, Domen K, Tatsumi T, Hara M, Hayashi S (2006) Acid-catalyzed reactions on flexible polycyclic aromatic carbon in amorphous carbon. Chem Mater 18:3039–3045. doi:10.1021/cm0605623
Okuhara T (2002) Water-tolerant solid acid catalysts. Chem Rev 102:3641–3666. doi:10.1021/cr0103569
Okuhara T, Mizuno N, Misono M (1996) Catalytic chemistry of heteropoly compounds. Adv Catal 41:113–252. doi:10.1016/S0360-0564(08)60041-3
Olah GA (1973) Friedel-crafts chemistry. Wiley, Hoboken
Olah GA, Prakash GKS, Sommer J (1985) Superacids. Wiley-Interscience, Hoboken
Pope MT (1983) Heteropoly and isopoly oxometalates (inorganic chemistry concepts vol. 8). Springer, Berlin
Rocchiccioli-Deltcheff C, Amirouche M, Fournier M (1992) Structure and catalytic properties of silica-supported polyoxomolybdates III. 12-molybdosilicic acid catalysts: vibrational study of the dispersion effect and nature of the Mo species in interaction with the silica support. J Catal 138:445–456. doi:10.1016/0021-9517(92)90296-T
Sartori G, Maggi R (2006) Use of solid catalysts in Friedel-Crafts acylation reactions. Chem Rev 106:1077–1104. doi:10.1021/cr040695c
Shirini F, Mamaghani M, Atghia SV (2011) Sulfonic acid-functionalized ordered nanoporous Na+-montmorillonite (SANM): A novel, efficient and recyclable catalyst for the chemoselective N-Boc protection of amines in solventless media. Catal Commun 12:1088–1094. doi:10.1016/j.catcom.2011.03.030
Song IK, Kaba MS, Barteau MA (2002) Nanoscale investigation of mixed arrays of Keggin-type and Wells-Dawson-type heteropolyacids (HPAs) by scanning tunneling microscopy (STM). Langmuir 18:2358–2362. doi:10.1021/la0111811
Song X, Zhu W, Yan Y, Gao H, Gao W, Zhang W, Jia M (2016) Selective oxidation of olefins with aqueous hydrogen peroxide over phosphomolybdic acid functionalized knitting aryl network polymer. J Mol Catal A: Chem 413:32–39. doi:10.1016/j.molcata.2015.12.012
Tan G, Li Z (2012) Highly active, stable, and recyclable magnetic nano-size solid acid catalysts: efficient esterification of free fatty acid in grease to produce biodiesel. Green Chem 14:3077–3086. doi:10.1039/C2GC35779H
Tao H, Yang H, Zhang Y, Ren J, Liu X, Wang Y, Lu G (2013) Space-confined synthesis of nanorod oriented-assembled hierarchical MFI zeolite microspheres. J Mater Chem A 1:13821–13827. doi:10.1039/C3TA12989F
Te M, Fairbridge C, Ring Z (2001) Oxidation reactivities of dibenzothiophenes in polyoxometalate/H2 O2 and formic acid/H2 O2 systems. Appl Catal A 219:267–280. doi:10.1016/S0926-860X(01)00699-8
Toda M, Takagaki A, Okamura M, Kondo JN, Hayashi S, Domen K, Hara M (2005) Green chemistry: biodiesel made with sugar catalyst. Nature 438:178. doi:10.1038/438178a
Venier CG, Squires TG, Chen YY, Smith BF (1982) Peroxytrifluoroacetic acid oxidation of sulfides to sulfoxides and sulfones. J Org Chem 47:3773–3774. doi:10.1021/jo00140a040
Voutyritsa E, Triandafillidi I, Kokotos CG (2017) Green Organocatalytic Oxidation of Sulfides to Sulfoxides and Sulfones. Synthesis 49:917–924. doi:10.1055/s-0036-1588315
Wang P, Wang X, Jing X, Zhu G (2000) Sol–gel-derived, polishable, 1: 12-phosphomolybdic acid-modified ceramic-carbon electrode and its electrocatalytic oxidation of ascorbic acid. Anal Chim Acta 424:51–56. doi:10.1016/S0003-2670(00)01134-X
Wang X, Chen CC, Chen SY, Mou Y, Cheng S (2005) Arenesulfonic acid functionalized mesoporous silica as a novel acid catalyst for the liquid phase Beckmann rearrangement of cyclohexanone oxime to ɛ-caprolactam. Appl Catal A 281:47–54. doi:10.1016/j.apcata.2004.11.011
Wang L, Zhang J, Yang S, Sun Q, Zhu L, Wu Q, Zhang H, Meng X, Xiao F-S (2013) Sulfonated hollow sphere carbon as an efficient catalyst for acetalisation of glycerol. J Mater Chem A 1:9422–9426. doi:10.1039/C3TA10956A
Wang B, Zhang J, Zou X, Dong H, Yao P (2015) Selective oxidation of styrene to 1, 2-epoxyethylbenzene by hydrogen peroxide over heterogeneous phosphomolybdic acid supported on ionic liquid modified MCM-41. Chem Eng J 260:172–177. doi:10.1016/j.cej.2014.08.076
Wilson K, Clark JH (2000) Solid acids and their use as environmentally friendly catalysts in organic synthesis. Pure Appl Chem 72:1313–1319. doi:10.1351/pac200072071313
Xi J, Zhang Y, Xia Q, Liu X, Ren J, Lu G, Wang Y (2013) Direct conversion of cellulose into sorbitol with high yield by a novel mesoporous niobium phosphate supported Ruthenium bifunctional catalyst. Appl Catal A 459:52–58. doi:10.1016/j.apcata.2013.03.047
Zhang Y, Wang J, Ren J, Liu X, Li X, Xia Y, Lu G, Wang Y (2012) Mesoporous niobium phosphate: an excellent solid acid for the dehydration of fructose to 5-hydroxymethylfurfural in water. Catal Sci Technol 2:2485–2491. doi:10.1039/C2CY20204B
Zhao J, Li B, Onda K, Feng M, Petek H (2006) Solvated electrons on metal oxide surfaces. Chem Rev 106:4402–4427. doi:10.1021/cr050173c
Zhao S, Cheng M, Li J, Tian J, Wang X (2011) One pot production of 5-hydroxymethylfurfural with high yield from cellulose by a Brønsted–Lewis–surfactant-combined heteropolyacid catalyst. Chem Commun 47:2176–2178. doi:10.1039/C0CC04444J
Zhu Y, Stubbs LP, Ho F, Liu R, Ship CP, Maguire JA, Hosmane NS Magnetic (2010) nanocomposites: a new perspective in catalysis. ChemCatChem 2:365–374. doi:10.1002/cctc.200900314
Acknowledgements
The authors would like to thank Semnan University Research Council for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zolfagharinia, S., Kolvari, E. & Koukabi, N. A practical heteropolyacid nanocatalyst supported on nano-sized ceramic for the chemoselective oxidation of sulfides to sulfoxides through an experimental design approach. Chem. Pap. 71, 2505–2520 (2017). https://doi.org/10.1007/s11696-017-0246-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-017-0246-0