Abstract
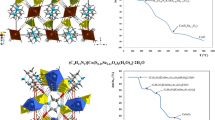
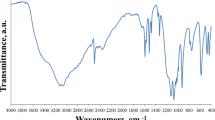
Two new co-based hybrid materials, (C4H12N2)[Co(H2O)6](S0.689Se0.311O4)2 (1) and (C4H12N2)[Co(H2O)6](S0.80Se0.20O4)2 (2), have been synthesized by slow evaporation method at room temperature and crystallographically characterized. They crystallizes isotypically in the monoclinic system, space group C2/c, with the following unit-cell parameters: a = 14.2559(19), b = 9.5066(13), c = 13.6759(19) Å, β = 114.930(4)°, V = 1680.7(4) Å3, and Z = 4 for (1) and a = 14.2235(19), b = 9.4901(14), c = 13.666(2) Å, β = 114.863(4)°, V = 1673.7(4) Å3, and Z = 4 for (2). Their crystal structure consist of metallic cations octahedrally coordinated by six water molecules [Co(H2O)6]2+, piperazinediium cations (C4H12N2)2+, and sulfate/selenate anions (S x Se1-x O4)2− linked together via two types of hydrogen bonds, Ow–H···O and N–H···O. The IR spectroscopic study confirms not only the substitution of the sulfur by the selenium but also the piperazine protonation. Through this work, it is demonstrated that the substitution of sulfur by selenium atom can lead to strong modification in the space group and atomic arrangement. Indeed the mixed selenate/sulfate compounds adopt a crystal structure different from those of the pure sulfate or selenate phases. The thermogravimetric measurement indicates that the dehydration occurs in two steps by the departure of six water molecules giving rise to an anhydrous phase. The final product of the decomposition is cobalt oxide, CoO, in both cases.
Graphical Abstract











Similar content being viewed by others
References
Barna E, Bommer B, Kursteiner J, Vital A, Trzebiatowski OV, Koch W, Schmid B, Graule T (2005) Innovative, scratch proof nanocomposites for clear coatings. Compos Part A 36:473–480. doi:10.1016/j.compositesa.2004.10.014
Bataille T, Louër D (2002) Two new diamine templated lanthanum sulfates, La2(H2O)2(C4H12N2)(SO4)4 and La2(H2O)2(C2H10N2)3(SO4)6·4H2O, with 3D and 2D crystal structures. J Mater Chem 12:3487–3493. doi:10.1039/B207212M
Bataille T, Louër D (2004) New linear and layered amine-templated lanthanum sulfates. J Solid State Chem 177:1235–1243. doi:10.1016/j.jssc.2003.10.031
Ben Hassan D, Rekik W, Naïli H, Mhiri T (2014) A new organically templated magnesium sulfate: structure, spectroscopic analysis, and thermal behaviour. Chem Pap 68:210–216. doi:10.2478/s11696-013-0432-7
Bhattacharya S, Dastidar P, Guru Row TN (1994) Hydrogen-bond-directed self-assembly of d-(+)-dibenzoyltartaric acid and 4-aminopyridine: optical nonlinearities and stoichiometry-dependent novel structural features. Chem Mater 6:531–537. doi:10.1021/cm00040a031
Brandenburg K (2004) Diamond, version 3.2i. Crystal Impact GbR, Bonn
Bruker-AXS (2014) SAINT Version 8.34A. Bruker AXS Inc., Madison
Cheetham AK, Férey G, Loiseau T (1999) Open-framework inorganic materials. Angew Chem Int Ed 38:3268–3292. doi:10.1002/(SICI)1521-3773(19991115)38:22<3268:AID-ANIE3268>3.0.CO;2-U
Dan M, Behera JN, Rao CNR (2004) Organically templated rare earth sulfates with three-dimensional and layered structures. J Mater Chem 14:1257–1265. doi:10.1039/B314663D
Daniel M-C, Astruc D (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104:293–346. doi:10.1021/cr030698+
Dastidar P, Guru Row TN, Prasad BR, Subramanian CK, Bhattacharya S (1993) Binary salts of substituted pyridines and l-tartaric acid as nonlinear optical organic materials: crystal structure of l-tartaric acid-4-dimethylaminopyridine (1:1) dihydrate salt. J Chem Soc Perkin Trans 2:2419–2422. doi:10.1039/P29930002419
Doran M, Norquist AJ, Hare DO (2002) The first organically templated actinide sulfate with a three dimensional framework structure. Chem Commun 26:2946–2947. doi:10.1039/B210272B
Galesié N, Jordanovsk VB (1992) Structures of dimethylammonium metal(III) sulfate hexahydrates (metal = Al, Cr). Acta Crystallogr Sect C 48:256–258. doi:10.1107/S0108270191009435
Holden AN, Matthias BT, Merz WJ, Remeika JP (1955) New class of ferroelectrics. Phys Rev 98:546. doi:10.1103/PhysRev.98.546
Hugh-Jones DA, Woodland AB, Angel RJ (1994) The structure of high-pressure C2/c ferrosilite and crystal chemistry of high-pressure C2/c pyroxene. Am Miner 79:1032–1041 (0003-004v94/I I I 2-1032$02.00)
Kadirvelraj R, Umarji AM, Robinson WT, Bhattacharya S, Guru Row TN (1996) Systematic crystallographic investigation of hydrogen-bonded networks involving monohydrogen tartrate–amine complexes: potential materials for nonlinear optics. Chem Mater 8:2313–2323. doi:10.1021/cm960123b
Kadirvelraj R, Bhattacharya S, Guru Row TN (1998) Conserved hydrogen bonded tartrate frameworks: inclusion of amines and implications for second harmonic generation. J Inclusion Phenom Mol Recognit Chem 30:321–330. doi:10.1023/A:1007926112616
Kammoun O, Loulou Nkhili N, Rekik W, Naïli H, Mhiri T, Bataille T (2012) Synthesis, crystal structure and characterization of a new dabcodiium hexaaquacobalt(II) bis(Selenate), (C6H14N2) Co(H2O)6 (SeO4)2. Chem Crystallogr 42:103–110. doi:10.1007/s10870-011-0210-8
Kirpichnikov LF, Andreev EF, Ivanov NR, Shuvalov LA, Varikash VM (1988) Some physical-properties of a new dimethylaminaluminiumsulfate ferroelectric. Crystallogr Rep 33:1437–1440
Kirpichnikov LF, Shuvalov LA, Ivanov NR, Prosolov BN, Andreyev EF (1989) Ferroelectricity in the dimethylaminaluminiumsulphate crystal. Ferroelectrics 96:313–317. doi:10.1080/00150198908216791
Krogman KC, Druffel T, Sunkara MK (2005) Anti-reflective optical coatings incorporating nanoparticles. Nanotechnology 16:S338–S343. doi:10.1088/0957-4484/16/7/005
Loulou N, Rekik W, Naïli H, Mhiri T, Bataille T (2013) Piperazinediium diselenatohexaaquacobaltate(II) dihydrate (C4H12N2)[Co(SeO4)2(H2O)4]·2H2O. Solid State Phenom 194:171–174. doi:10.4028/www.scientific.net/SSP.194.171
Loulou N, Rekik W, Naïli H (2014) A new nickel selenate template by piperazine: chemical preparation, crystal structure, thermal decomposition and magnetic properties. Monatshafte für chemie 145:931–936
Morimoto CN, Lingafelter EC (1970) The crystal structure of zinc guanidinium sulfate. Acta Crystallogr B 26:335–341. doi:10.1107/S0567740870002364
Naïli H, Rekik W, Bataille T, Mhiri T (2006) Crystal structure, phase transition and thermal behaviour of dabcodiium hexaaquacopper(II) bis(sulfate), (C6H14N2)[Cu(H2O)6](SO4)2. Polyhedron 25:3543–3554. doi:10.1016/j.poly.2006.07.010
Nicole L, Roses L, Sanchez C (2011) Integrative approaches to hybrid multifunctional materials: from multidisciplinary research to applied technologies. Adv Mater 22:3208–3214. doi:10.1002/adma.201000231
Norquist A, Doran MB, Thomas PM, Hare DO (2003) Structural diversity in organically templated uranium sulfates. Dalton Trans 6:1168–1175. doi:10.1039/B209208E
Pan JX, Yang GY, Sun YQ (2003) Piperazinium hexaaquacobalt(II) disulfate. Acta crystallogr E59:m286–m288. doi:10.1107/S1600536803008407
Pietraszko A, Lukaszewicz K, Kirpichnikov LF (1993) Crystal-structures of (CH3)2NH2AL(SO4)2.6H2O,(CH3)2NH2GA(SO4)2.6H2O, and (CH3)2NH2Al(S0.89Se0.11O4)2.6H2O. Polish J Chem 67:1877–1884
Rekik W, Naïli H, Bataille T, Roisnel T, Mhiri T (2006) Supramolecular networks of transition metal sulfates templated by piperazine. Inorg Chim Acta 359:3954–3962. doi:10.1016/j.ica.2006.05.030
Rekik W, Naïli H, Mhiri T, Bataille T (2009) Ethylenediammonium tetraaquadisulfatomagnesium(II). Acta Crystallogr E 65:1404–1405. doi:10.1107/S1600536809041981
Rekik W, Naïli H, Mhiri T, Bataille T (2011) Ethylenediammonium tetraaquadisulfatocadmate: ethylenediammonium tetraaquadisulfatocadmate. Acta Crystallogr E67:1176–1177. doi:10.1107/S1600536811030005
Ruiz-Valero C, Cascales C, Gomez-Lor B, Gutierrez-Puebla E, Iglesias M, Angeles Monge M, Snejko N (2002) New catalytically active neodymium sulfate. J Mater Chem 12:3073–3077. doi:10.1039/B205215F
Sanchez C (2005) Application of hybrid organic-inorganic nanonocomposites. J Mater Chem 15:3559–3592. doi:10.1039/B509097K
Sanchez C, Belleville P, Popall M, Nicole L (2011) Applications of advanced hybrid organic-inorganic nanomaterials: from laboratory to market. Chem Soc Rev 40:696–753. doi:10.1039/C0CS00136H
Schotner G, Rose K, Posset U (2003) Scratch and abrasion resistant coatings on plastic lenses-state of the art, developments and perspectives. J Sol Gel Sci Technol 27:71–79. doi:10.1023/A:1022684011222
Sheldrick GM (2014) SADABS version 2014/5. Bruker AXS Inc., Madison
Sheldrick GM (2015a) SHELXT—integrated space-group and crystal-structure determination. Acta Crystallogr A 71:3–8. doi:10.1107/S2053273314026370
Sheldrick GM (2015b) Crystal structure refinement with SHELXL. Acta Crystallogr C 71:3–8. doi:10.1107/S2053229614024218
Soloukhin VA, Posthums W, Brokken-Zijp JCM, Loos J, De with G (2002) Mechanical properties of silica-(meth(acrylate) hybrid coatings on polycarbonate substrate. Polymer 43:6169–6181. doi:10.1016/S0032-3861(02)00542-6
Takahashi S, Goldberg HA, Feeney CA, Kaim DP, Farrel MO, Leary K, Paul DR (2006) Gas barrier properties of butyl rubber/vermiculite nanocomposite coatings. Polymer 47:3083–3093. doi:10.1016/j.polymer.2006.02.077
Wu LYL, Chwa E, Chen Z, Zeng XTA (2008) Study towards improving mechanical properties of sol gel coatings for polycarbonate. Thin Solid Films 516:1056–1062. doi:10.1016/j.tsf.2007.06.149
Xing Y, Liu Y, Shi Z, Meng H, Pang W (2003a) Synthesis and structure of organically templated lanthanum sulfate [C4N3H16][La(SO4)3]·H2O. J Solid State Chem 174:381–385. doi:10.1016/S0022-4596(03)00255-X
Xing Y, Shi Z, Li G, Pang W (2003b) Hydrothermal synthesis and structure of [C2N2H10][La2(H2O)4(SO4)4]·2H2O, a new organically templated rare earth sulfate with a layer structure. Dalton Trans 5:940–943. doi:10.1039/B211076H
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kadri, I., Derbel, M.A., Naïli, H. et al. Effect of the partial substitution of sulfur by selenium on the atomic arrangement in sulfate- and selenate-based compounds. Chem. Pap. 71, 2063–2073 (2017). https://doi.org/10.1007/s11696-017-0199-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-017-0199-3