Abstract
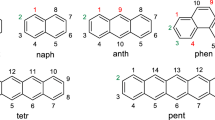
In a recent paper (Radenkovic et al. Chem Phys Lett 625:69–72, 2015), a new method for quantifying the strain energy in benzenoid molecules, resulting from the repulsion between the bay H-atoms was elaborated. In this work, we present a modified procedure, capable of estimating the strain energy in a single-step calculation. Strain energies were obtained at the B3LYP/def2-TZVP level of density functional theory. It was found that in benzenoid molecules with a single bay region, the strain energy is essentially constant, equal to around 7.3 kJ/mol. On the other hand, in the case of the first four members of the fibonacene series, the strain energy is found to be linearly proportional to the number of bay regions.


Similar content being viewed by others
References
Balaban AT (1989) Symmtery and enumeration of fibonacenes (unbranched catacondensed benzenoids isoarithmic with helicenes and zigzag catafusenes). MATCH Commun Math Chem 24:29–38
Balaban AT, Schmalz TG (2006) Strain-free sextet-resonant benzenoids and their antisextet dualists. J Chem Inf Model 46:1563–1579. doi:10.1021/ci060007l
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. doi:10.1063/1.464913
Clar E (1972) The aromatic sextet. Wiley, London
Cruz R, Gutman I, Rada J (2012) Convex hexagonal systems and their topological indices. MATCH Commun Math Comput Chem 68:97–108
Cruz R, Gutman I, Rada J (2013) On benzenoid systems with a minimal number of inlets. J Serb Chem Soc 78:1351–1357. doi:10.2298/JSC130218033C
Dias JR (1987) Handbook of polycyclic hydrocarbons. Part A. Benzenoid hydrocarbons. Elsevier, Amsterdam, pp 86–96
Dias JR (2004) The most stable class of benzenoid hydrocarbons—New topological correlations of strain-free total resonant sextet benzenoids. J Chem Inf Comput Sci 44:1210–1220. doi:10.1021/ci049910g
Dias JR (2007) Strain-free total resonant sextet benzenoids and their antisextet dualists and retro-leapfrogs. J Chem Inf Model 47:20–24. doi:10.1021/ci600371h
Dias JR (2010) The polyhex/polypent topological paradigm: regularities in the isomer numbers and topological properties of select subclasses of benzenoid hydrocarbons and related systems. Chem Soc Rev 39:1913–1924. doi:10.1039/b913686j
Dias JR, Cyvin SJ, Brunvoll J (1991) Strain-free total resonant sextet benzenoid hydrocarbons. Polyc Arom Comp 2:95–208. doi:10.1080/10406639108048940
Ferao AE (2016) Kinetic energy density per electron as quick insight into ring strain energies. Tetrahedron Lett 57:5616–5619. doi:10.1016/j.tetlet.2016.10.115
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Rev A.1. Gaussian Inc., Wallingford
Goddard R, Haenel MW, Herndon WC, Krüger C, Zander M (1995) Crystallization of large planar polycyclic aromatic hydrocarbons: the molecular and crystal structures of hexabenzo[bc, ef, hi, kl, no, qr]coronene and benzo[1,2,3-bc:4,5,6-b′c′]dicoronene. J Am Chem Soc 117:30–41. doi:10.1021/ja00106a004
Gutman I, Cyvin SJ (1989) Introduction to the theory of benzenoid hydrocarbons. Springer, Berlin, pp 73–76
Gutman I, Klavžar S (2006) Chemical graph theory of fibonacenes. MATCH Commun Math Chem 55:39–54
Gutman I, Balaban AT, Randić M, Kiss-Tóth C (2005) Partitioning of π-electrons in rings of fibonacenes. Z Naturforsch 60a:171–176
Herndon WC, Párkányi C (1976) π bond orders and bond lengths. J Chem Educ 53:689–692. doi:10.1021/ed053p689
Herndon WC, Norwalk PC, Connor DA, Lin P (1992) Empirical model calculations for thermodynamic and structural properties of condensed polycyclic aromatic hydrocarbons. J Am Chem Soc 114:41–47. doi:10.1021/ja00027a005
Lazzarin P, Berto F (2005) Some expressions for the strain energy in a finite volume surrounding the root of blunt V-notches. Int J Fracture 135:161–185. doi:10.1007/s10704-005-3943-6
Lazzarin P, Filippi S (2006) A generalized stress intensity factor to be applied to rounded V-shaped notches. Int J Solids Stuct 43:2461–2478. doi:10.1016/j.ijsolstr.2005.03.007
Lazzarin P, Berto F, Zappalorto M (2010) Rapid calculations of notch stress intensity factors based on averaged strain energy density from coarse meshes: theoretical bases and applications. Int J Fatigue 32:1559–1567. doi:10.1016/j.ijfatigue.2010.02.017
Lee C, Yang W, Parr RG (1988) Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. doi:10.1103/PhysRevB.37.785
Mo Y, Zhang H, Su P, Yarowski PD, Wu W (2016) Intramolecular multi-bond strain: the unrecognized side of the dichotomy of conjugated systems. Chem Sci 7:5872–5878. doi:10.1039/C6SC00454G
Pauling L (1980) Bond numbers and bond lengths in tetrabenzo[de, no, st, c1d1]heptacene and other condensed aromatic hydrocarbons: a valence-bond treatment. Acta Cryst B 36:1898–1901. doi:10.1107/S056774088000742X
Pauling L, Brockway LO, Beach JY (1935) The dependence of interatomic distance on single bond-double bond resonance. J Am Chem Soc 57:2705–2709. doi:10.1021/ja01315a105
Pinter B, Fievez T, Bickelhaupt FM, Geerlings P, De Proft F (2012) On the origin of the steric effect. Phys Chem Chem Phys 14:9846–9854. doi:10.1039/C2CP41090G
Poater J, Visser R, Solà M, Bickelhaupt FM (2007) Polycyclic benzenoids: why kinked is more stable than straight. J Org Chem 72:1134–1142. doi:10.1021/jo061637p
Radenković S, Gutman I, Đorđević S (2015) Strain in strain-free benzenoid hydrocarbons: the case of phenanthrene. Chem Phys Lett 625:69–72. doi:10.1016/j.cplett.2015.02.039
Radenković S, Gutman I, Ružić S, Zdravković S (2016) Strain in strain-free benzenoid hydrocarbons: the case of chrysene and triphenylene. Rev RoumChim 61:261–267
Randić M (2003) Aromaticity of polycyclic conjugated hydrocarbons. Chem Rev 103:3449–3606. doi:10.1021/cr9903656
Suresh CH, Gadre SR (1999) Clar’s aromatic sextet theory revisited via molecular electrostatic potential topography. J Org Chem 64:2505–2512. doi:10.1021/jo990050q
Suresh CH, Koga N (2002) Accurate calculation of aromaticity of benzene and antiaromaticity of cyclobutadiene: New homodesmotic reactions. J Org Chem 67:1965–1968. doi:10.1021/jo010903q
Suresh CH, Koga N (2006) An isodesmic reaction based approach to aromaticity of a large spectrum of molecules. Chem Phys Lett 419:550–556. doi:10.1016/j.cplett.2005.12.028
Suresh CH, Lincy TL, Mohan N, Rakhi R (2015) Aromatization energy and strain energy of buckminsterfullerene from homodesmotic reactions. J Phys Chem A 119:6683–6688. doi:10.1021/acs.jpca.5b01157
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys Chem Chem Phys 7:3297–3305. doi:10.1039/B508541A
Wiberg KB (1986) The concept of strain in organic chemistry. Angew Chem Int Edit 25:312–322. doi:10.1002/anie.198603121
Acknowledgements
S. R. and M. A. thank the Serbian Ministry of Education and Science for partial support of this work through Grant No. 174033.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Radenković, S., Gutman, I., Zdravković, S. et al. Strain in strain-free benzenoid hydrocarbons: the case of fibonacenes. Chem. Pap. 71, 1491–1495 (2017). https://doi.org/10.1007/s11696-017-0143-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-017-0143-6