Abstract
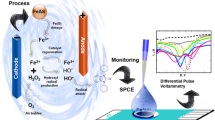
The electrodegradation of azithromycin was studied by its indirect oxidation using dimensionally stable Ti/RuO2 anode as catalyst in the electrolyte containing methanol, 0.05 M NaHCO3, sodium chloride and deionized water. The optimal conditions for galvanostatic electrodegradation for the azithromycin concentration of 0.472 mg cm−3 were found to be NaCl concentration of 7 mg cm−3 and the applied current of 300 mA. The differential pulse voltammetry using glassy carbon electrode was performed for the first time in the above-mentioned content of electrolyte for the nine concentration of azithromycin (0.075–0.675 mg cm−3) giving the limits of azithromycin detection and of quantification as: LOD 0.044 mg cm−3 and LOQ 0.145 mg cm−3. The calibration curve was constructed enabling the electrolyte analysis during its electrodegradation process. The electrolyte was analyzed by high-performance liquid chromatography and electrospray ionization time-of-flight mass spectrometry. The electrooxidation products were identified and after 180 min there was no azithromycin in the electrolyte while TOC analysis showed that 79% of azithromycin was mineralized. The proposed degradation scheme is presented.



Similar content being viewed by others
References
Abia L, Armesto XL, Canle LM, Garcia MV, Santaballa JA (1998) Oxidation of aliphatic amines by aqueous chlorine. Tetrahedron 54:521–530
Al-Rimawi F, Kharoaf M (2010) Analysis of azithromycin and its related compounds by RP-HPLC with UV detection. J Chromatogr Sci 48:86–90. doi:10.1093/chromsci/48.2.86
Avramov Ivic ML, Petrovic SD, Mijin DZ, Zivkovic PM, Kosovic IM, Drljevic KM, Jovanovic MB (2006) Studies on electrochemical oxidation of azithromycin and Hemomycin at gold electrode in neutral electrolyte. Electrochim Acta 51:2407–2416. doi:10.1016/j.electacta.2005.07.032
Avramov Ivic ML, Petrovic SD, Mijin DZ (2007) A study of the electrochemical activity of some macrolide antibiotics on a gold electrode in a neutral electrolyte. J Serb Chem Soc 72:1427–1436. doi:10.2298/JSC0712427A
Bessegato GG, Guaraldo TT, Ferreira de Brito J, Brugnera MF, Boldrin Zanoni MV (2015) Achievements and trends in photoelectrocatalysis: from environmental to energy applications. Electrocatalysis-US 6:415–441. doi:10.1007/s12678-015-0259-9
Brillas E, Martinez-Huitle CA (2015) Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl Catal B 166–167:603–643. doi:10.1016/j.apcatb.2014.11.016
Council of Europe (2014) European Pharmacopoeia. 8th edn. Strasbourg
da Silva OB, Machado SAS (2012) Evaluation of the detection and quantification limits in electroanalysis using two popular methods: application in the case study of paraquat determination. Anal Methods 4:2348–2354. doi:10.1039/c2ay25111f
Damjanović A, Zdunić G, Šavikin K, Mandić B, Jadranin M, Matić I, Stanojković T (2016) Evaluation of the anti-cancer potential of Mahonia aquifolium extracts via apoptosis and anti-angiogenesis. Bangladesh J Pharmacol 11:741–749. doi:10.3329/bjp.v11i3.27103
Deborde M, von Gunten U (2008) Reactions of chlorine with inorganic and organic compounds during water treatment—kinetics and mechanisms: a critical review. Water Res 42:13–51. doi:10.1016/j.watres.2007.07.025
Drljevic-Djuric KM, Jovic VD, Lacnjevac UC, Avramov Ivic ML, Petrovic SD, Mijin DZ, Djordjevic SB (2010) Voltammetric and differential pulse determination of roxithromycin. Electrochim Acta 56:47–52. doi:10.1016/j.electacta.2010.09.067
Ghari T, Kobarfard F, Mortazavi S (2013) Development of a simple RP-HPLC-UV method for determination of azithromycin in pharmaceutical dosage forms as an alternative to the USP method. Iran J Pharm Res 12:57–63
Glavaški OS, Petrović SD, Mijin DZ, Jovanović MB, Dugandžić AM, Zeremski TM, Avramov Ivić ML (2014) Electrochemical degradation of the pesticide dimethenamid-P at gold, DSA platinum and ruthenium oxide electrodes in different electrolytes. Electroanalysis 26:1877–1880. doi:10.1002/elan.201400249
Kummerer K (2009) Antibiotics in the aquatic environment—a review—part II. Chemosphere 75:435–441. doi:10.1016/j.chemosphere.2008.12.006
Kwiecień A, Krzek J (2012) Stress degradation studies on azithromycin and development of a validated stability-indicating TLC-densitometric method with HPLC/electrospray ionization-MS analysis of degradation products. J AOAC Int 95:1418–1428. doi:10.5740/jaoacint.10-196
Li X, Peng H, Tian B, Gou J, Yao Q, Tao X, He H, Zhang Y, Tang X, Cai C (2015) Preparation and characterization of azithromycin–Aerosil 200 solid dispersions with enhanced physical stability. Int J Pharm 486:175–184. doi:10.1016/j.ijpharm.2015.03.029
Mandic Z, Weitner Z, Ilijas M (2003) Electrochemical oxidation of azithromycin and its derivatives. J Pharm Biomed Anal 33:647–654. doi:10.1016/S0731-7085(03)00315-7
Martínez-Huitle CA, Rodrigo MA, Sirés I, Scialdone O (2015) Single and coupled electrochemical processes and reactors for the abatement of organic water pollutants: a critical review. Chem Rev 115:13362–13407. doi:10.1021/acs.chemrev.5b00361
Mijin DZ, Avramov Ivić ML, Onjia AE, Grgur BN (2012) Decolorization of textile dye CI Basic Yellow 28 with electrochemically generated active chlorine. Chem Eng J 204–206:151–157. doi:10.1016/j.cej.2012.07.091
Mojica ERE, Aga DS (2011) Antibiotics pollution in soil and water: potential ecological and human health issues. In: Nriagu JO (ed) Encyclopedia of environmental health, 1st edn. Elsevier, Burlington, pp 97–110
Nigovic B (2004) Adsorptive stripping voltammetric determination of azithromycin by glassy carbon electrode modified electrochemical oxidation. Anal Sci 20:639–643. doi:10.2116/analsci.20.639
Nigovic B, Simunic B (2003) Determination of 5-aminosalicylic acid in pharmaceutical formulation by differential pulse voltammetry. J Pharm Biomed Anal 32:197–202. doi:10.1016/S0731-7085(03)00060-8
Oturan N, Wu J, Zhang H, Sharma VK, Oturan MA (2013) Electrocatalytic destruction of the antibiotic tetracycline in aqueous medium by electrochemical advanced oxidation processes: Effect of electrode materials. Appl Catal B 140–141:92–97. doi:10.1016/j.apcatb.2013.03.035
Pacheco SR, da Cunha FJB, Faria AF (2014) Azithromycin drag determination in pharmaceutical formulations by UV spectrometry and HPLC–UV. Braz J Anal Chem 3:481–486
Peng JY, Hou CT, Liu XX, Li HB, Hu XY (2011) Electrochemical behavior of azithromycin at graphene and ionic liquid composite film modified electrode. Talanta 86:227–232. doi:10.1016/j.talanta.2011.09.005
Sato K, Takasugi M (2000) Physicochemical properties and stability of the 15-membered macrolide azithromycin. Kagaku Ryoho no Ryouiki 16:1170–1174
Shawabkeh RA, Tutunji MF (2002) Mathematical modeling of the electrode process of azithromycin using cyclic voltammetry at hanging mercury drop electrode. Sensors-Basel 2:436–446. doi:10.3390/s21100436
Slepchenko ES, Karbainov YA, Slepchenko GB, Anisimova LS (2004) Voltammetric research of electrochemical activity of azithromycin dehydrate. Izv Vyssh Uchebn Zaved, Khim Khim Tekhnol 47:6–9
Timoumi S, Mangin D, Peczalski R, Zagrouba F, Andrieu J (2014) Stability and thermophysical properties of azithromycin dehydrate. Arab J Chem 7:189–195. doi:10.1016/j.arabjc.2010.10.024
Tong L, Eichhorn P, Perez S, Wang Y, Barcelo D (2011) Photodegradation of azithromycin in various aqueous systems under simulated and natural solar radiation: kinetics and identification of photoproducts. Chemosphere 83:340–348. doi:10.1016/j.chemosphere.2010.12.025
Verbruggen SW, Tytgat T, Van Passel S, Martens JA, Lenaerts S (2014) Cost-effectiveness analysis to assess commercial TiO2 photocatalysts for acetaldehyde degradation in air. Chem Pap 68:1273–1278. doi:10.2478/s11696-014-0557-3
Wahyuningsih S, Purnawan C, Kartikasari PA, Praistia N (2014) Visible light photoelectrocatalytic degradation of rhodamine B using a dye-sensitised TiO2 electrode. Chem Pap 68:1248–1256. doi:10.2478/s11696-013-0476-8
Wang Z, Song X, Zhou T, Bian K, Zhang F, He L, Liua Q (2015) Simultaneous determination of ten macrolides drugs in feeds by high performance liquid chromatography with evaporation light scattering detection. RSC Adv 5:1491–1499. doi:10.1039/C4RA12623H
Wu Y, Ji X, Hu S (2004) Studies on electrochemical oxidation of azithromycin and its interaction with bovine serum albumin. Bioelectrochemistry 64:91–97. doi:10.1016/j.bioelechem.2004.03.005
Zhang Y, Liu X, Cui Y, Huang H, Chi N, Tang X (2009) Aspects of degradation kinetics of azithromycin in aqueous solution. Chromatographia 70:67–73. doi:10.1365/s10337-009-1116-x
Acknowledgements
The work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant Nos. ON172013 and ON172060).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Radosavljević, K.D., Lović, J.D., Mijin, D.Ž. et al. Degradation of azithromycin using Ti/RuO2 anode as catalyst followed by DPV, HPLC–UV and MS analysis. Chem. Pap. 71, 1217–1224 (2017). https://doi.org/10.1007/s11696-016-0115-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-016-0115-2