Abstract
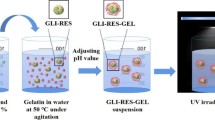
Finding new, biocompatible matrices that allow us to model the generation of free radicals is of utmost importance for balancing the harmful and beneficial effects of the latter. In this respect, we report here the simultaneous encapsulation of the radical source and the antioxidant agent in a polyethylene glycol/β-cyclodextrin (PEG/β-CD) covalent gel network. We used electron paramagnetic resonance spectroscopy to evaluate the scavenging action of plant extracts (purple loosestrife, comfrey, milfoil, horsetail, thyme, carob, green coffee) embedded in PEG/β-CD gels. Free radicals were generated in situ by UV irradiation of riboflavin co-embedded in the gels. Prior to this, the extracts were characterized in what concerns their antioxidant activity, and their major polyphenolic constituents were quantified by liquid chromatography-electrospray ionization-tandem mass spectrometry. Purple loosestrife showed the highest antioxidant capacity, followed by comfrey and milfoil. Using the 5,5-dimethyl-1-pyrroline N-oxide spin trap, we have demonstrated that the gel-embedded extracts effectively scavenge the reactive carbon-centered free radicals generated in gel. The PEG/β-CD gels have been shown to be a valuable alternative matrix for the encapsulation of plant active principles having antioxidant activity. Moreover, co-encapsulation of the radical source transforms these gels into a controlled environment in which free radical processes can be tailored.




Similar content being viewed by others
References
Abd El-Ghaffar MA, Hashem MS, El-Awady MK, Rabie AM (2012) pH-sensitive sodium alginate hydrogels for riboflavin controlled release. Carbohydr Polym 89:667–675. doi:10.1016/j.carbpol.2012.03.074
Andrés-Lacueva C, Mattivi F, Tonon D (1998) Determination of riboflavin, flavin mononucleotide and flavin-adenine dinucleotide in wine and other beverages by high-performance liquid chromatography with fluorescence detection. J Chromatogr A 823:355–363. doi:10.1016/S0021-9673(98)00585-8
Bakaic E, Smeets NMB, Hoare T (2015) Injectable hydrogels based on poly(ethylene glycol) and derivatives as functional biomaterials. RSC Adv 5:35469–35486. doi:10.1039/C4RA13581D
Belščak-Cvitanović A, Stojanović R, Manojlović V, Komes D, Juranović Cindrić I, Nedović V, Bugarski B (2011) Encapsulation of polyphenolic antioxidants from medicinal plant extracts in alginate–chitosan system enhanced with ascorbic acid by electrostatic extrusion. Food Res Int 44:1094–1101. doi:10.1016/j.foodres.2011.03.030
Betz M, Steiner B, Schantz M, Oidtmann J, Mäder K, Richling E, Kulozik U (2012) Antioxidant capacity of bilberry extract microencapsulated in whey protein hydrogels. Food Res Int 47:51–57. doi:10.1016/j.foodres.2012.01.010
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 28:25–30. doi:10.1016/S0023-6438(95)80008-5
Buettner G (1987) Spin trapping: ESR parameters of spin adducts. Free Radic Biol Med 3:259–303. doi:10.1016/S0891-5849(87)80033-3
Burda S, Oleszek W (2001) Antioxidant and antiradical activities of flavonoids. J Agric Food Chem 49:2774–2779. doi:10.1021/jf001413m
Cesteros LC, Ramirez CA, Peciña A, Katime I (2006) Poly(ethylene glycol-β-cyclodextrin) gels: synthesis and properties. J Appl Poly Sci 102:1162–1166. doi:10.1002/app.24390
Cesteros LC, Ramirez CA, Peciña A, Katime I (2007) Synthesis and properties of hydrophilic networks based on poly(ethylene glycol) and β-cyclodextrin. Macromol Chem Phys 208:1764–1772. doi:10.1002/macp.200700109
Chatterjee D, Bhattacharjee P (2013) Comparative evaluation of the antioxidant efficacy of encapsulated and un-encapsulated eugenol-rich clove extracts in soybean oil: shelf-life and frying stability of soybean oil. J Food Eng 117:545–550. doi:10.1016/j.jfoodeng.2012.11.016
Constantin MM, Corbu C, Ionita G (2010) EPR study on the role of riboflavin used in photo-oxidative collagen cross-linking. Rev Chim 61:495–497
Crini G (2014) Review: a history of cyclodextrins. Chem Rev 114:10940–10975. doi:10.1021/cr500081p
Detoni CB, Cabral-Albuquerque ECM, Hohlemweger SVA, Sampaio C, Barros TF, Velozo ES (2009) Essential oil from Zanthoxylum tingoassuiba loaded into multilamellar liposomes useful as antimicrobial agents. J Microencaps 26:684–691. doi:10.1080/02652040802661887
Diakonis VF, Grentzelos MA, Tzatzarakis MN, Kankaria V, Karavitaki A, Karatapanis AE, Tsatsakis AM, Kymionis GD (2012) Riboflavin’s time-dependent degradation rate induced by ultraviolet A irradiation. Eur J Ophthalmol 22:S51–S56. doi:10.5301/ejo.5000114
Dröge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82:47–95. doi:10.1152/physrev.00018.2001
Duling DR (1994) Simulation of multiple isotropic spin trap EPR spectra. J Magn Reson B 104:105–110. doi:10.1006/jmrb.1994.1062
Duling DR (1996) PEST Winsim, version 0.96. National Institute of Environmental Health Sciences, Triangle Park, NC
Eichler M, Lavi R, Shainberg A, Lubart R (2005) Flavins are source of visible-light-induced free radical formation in cells. Lasers Surg Med 37:314–319. doi:10.1002/lsm.20239
Fang Z, Bhandari B (2010) Encapsulation of polyphenols—a review. Trends Food Sci Tech 21:510–523. doi:10.1016/j.tifs.2010.08.003
Foti MC, Daquino C, Geraci C (2004) Electron-transfer reaction of cinnamic acids and their methyl esters with the DPPH• radical in alcoholic solutions. J Org Chem 69:2309–2314. doi:10.1021/jo035758q
Grzelak A, Rychlik B, Bartosz G (2001) Light-dependent generation of reactive oxygen species in cell culture media. Free Rad Biol Med 30:1418–1425. doi:10.1016/S0891-5849(01)00545-7
Heelis PF, Koziolowa A (1991) The effect of hydrogen bonding on the electron transfer reactions of the excited singlet and triplet states of flavins. J Photochem Photobiol B Biol 11:365–370. doi:10.1016/1011-1344(91)80041-F
Ionita G, Chechik V (2010) Exploring polyethylene glycol/cyclodextrin hydrogels with spin probes and EPR spectroscopy. Chem Commun 46:8255–8257. doi:10.1039/C0CC02759F
Ionita G, Ariciu AM, Turcu IM, Chechik V (2014) Properties of polyethylene glycol/cyclodextrin hydrogels revealed by spin probes and spin labelling methods. Soft Matter 10:1778–1783. doi:10.1039/C3SM52004H
Ionita P, Dinoiu V, Munteanu C, Turcu IM, Tecuceanu V, Zaharescu T, Oprea E, Ilie C, Anghel D, Ionita G (2015) Antioxidant activity of rosemary extracts in solution and embedded in polymeric systems. Chem Pap 69:872–880. doi:10.1515/chempap-2015-0024
Iuga C, Alvarez-Idaboy JR, Russo N (2012) Antioxidant activity of trans-resveratrol toward hydroxyl and hydroperoxyl radicals: a quantum chemical and computational kinetics study. J Org Chem 77:3868–3877. doi:10.1021/jo3002134
Kedare SB, Singh RP (2011) Genesis and development of DPPH method of antioxidant assay. J Food Sci Technol 48:412–422. doi:10.1007/s13197-011-0251-1
Kowalczyk RM, Schleicher E, Bittl R, Weber S (2004) The photoinduced triplet of flavins and its protonation states. J Am Chem Soc 126:11393–11399. doi:10.1021/ja049554i
Laane C, de Roo G, van den Ban E, Sjauw-En-Wa MW, Duyvis MG, Hagen WA, van Berkel WJH, Hilhorst R, Schmedding DJM, Evans DJ (1999) The role of riboflavin in beer flavour instability: EPR studies and the application of flavin binding proteins. J Inst Brew 105:392–397. doi:10.1002/j.2050-0416.1999.tb00031.x
Leopoldini M, Marino T, Russo N, Toscano M (2004) Antioxidant properties of phenolic compounds: H-atom versus electron transfer mechanism. J Phys Chem A 108:4916–4922. doi:10.1021/jp037247d
Leopoldini M, Russo N, Toscano M (2011) The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem 125:288–306. doi:10.1016/j.foodchem.2010.08.012
Li M, Tshabalala MA, Buschle-Diller G (2016) Formulation and characterization of polysaccharide beads for controlled release of plant growth regulators. J Mater Sci 51:4609–4617. doi:10.1007/s10853-016-9775-0
Liu F, Gu H, Lin Y, Qi Y, Dong X, Gao J, Cai T (2012) Surface-enhanced Raman scattering study of riboflavin on borohydride-reduced silver colloids: dependence of concentration, halide anions and pH values. Spectrochim Acta A Mol Biomol Spectrosc 85:111–119. doi:10.1016/j.saa.2011.09.043
Lupo B, Maestro A, Gutierrez JM, Gonzalez C (2015) Characterization of alginate beads with encapsulated cocoa extract to prepare functional food: comparison of two gelation mechanisms. Food Hydrocoll 49:25–34. doi:10.1016/j.foodhyd.2015.02.023
Mabry TJ, Markham KR, Thomas MB (1970) The systematic identification of flavonoids. Springer-Verlag, New York
Masuda T, Yamada K, Akiyama J, Someya T, Odaka Y, Takeda Y, Tori M, Nakashima K, Maekawa T, Sone Y (2008) Antioxidation mechanism studies of caffeic acid: identification of antioxidation products of methyl caffeate from lipid oxidation. J Agric Food Chem 56:5947–5952. doi:10.1021/jf800781b
Munin A, Edwards-Levy F (2011) Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics 3:793–829. doi:10.3390/pharmaceutics3040793
Ozcelik B, Lee JH, Min DB (2003) Effects of light, oxygen, and pH on the absorbance of 2,2-diphenyl-1-picrylhydrazyl. J Food Sci 68:487–490. doi:10.1111/j.1365-2621.2003.tb05699.x
Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK, Levine M (2003) Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr 22:18–35. doi:10.1080/07315724.2003.10719272
Polyakov NE, Kruppa AI, Leshina TV, Konovalova TA, Kispert LD (2001) Carotenoids as antioxidants: spin trapping EPR and optical study. Free Rad Biol Med 31:43–52. doi:10.1016/S0891-5849(01)00547-0
Pyrzynska K, Pekal A (2013) Application of free radical diphenylpicrylhydrazyl (DPPH) to estimate the antioxidant capacity of food samples. Anal Method 5:4288–4295. doi:10.1039/C3AY40367J
Rice-Evans CA, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Rad Biol Med 20:933–956. doi:10.1016/0891-5849(95)02227-9
Sanna D, Delogu G, Mulas M, Schirra M, Fadda A (2012) Determination of free radical scavenging activity of plant extracts through DPPH assay: an EPR and UV–Vis study. Food Anal Method 5:759–766. doi:10.1007/s12161-011-9306-1
Sel S, Nass N, Pötzsch S, Trau S, Simm A, Kalinski T, Duncker GI, Kruse FE, Auffarth GU, Brömme HJ (2014) UVA irradiation of riboflavin generates oxygen-dependent hydroxyl radicals. Redox Rep 19:72–79. doi:10.1179/1351000213Y.0000000076
Singleton VL, Rosi JA Jr (1965) Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Soussan E, Cassel S, Blanzat M, Rico-Lattes I (2009) Drug delivery by soft matter: matrix and vesicular carriers. Angew Chem Int Ed 48:274–288. doi:10.1002/anie.200802453
Stojanovic R, Belscak-Cvitanovic A, Manojlovic V, Komes D, Nedovic V, Bugarskia B (2012) Encapsulation of thyme (Thymus serpyllum L.) aqueous extract in calcium alginate beads. J Sci Food Agric 92:685–696. doi:10.1002/jsfa.4632
Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84. doi:10.1016/j.biocel.2006.07.001
Wand K, Neuhann R, Ullmann A, Plank K, Baumann M, Ritter R, Griffith M, Lohmann CP, Kobuch K (2015) Riboflavin-UV-A crosslinking for fixation of biosynthetic corneal collagen implants. Cornea 34:544–549. doi:10.1097/ICO.0000000000000399
Wichchukit S, Oztop MH, McCarthy MJ, McCarthy KL (2013) Whey protein/alginate beads as carriers of a bioactive component. Food Hydrocoll 33:66–73. doi:10.1016/j.foodhyd.2013.02.013
Wollensak G, Spoerl E, Seiler T (2003) Stress–strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract Surg 29:1780–1785. doi:10.1016/S0886-3350(03)00407-3
Xie J, Schaich KM (2014) Re-evaluation of the 2,2-diphenyl-1-picrylhydrazyl free radical (DPPH) assay for antioxidant activity. J Agric Food Chem 62:4251–4260. doi:10.1021/jf500180u
Yang B, Wang JS, Zhao MM, Liu Y, Guang W, Jiang YM (2006) Identification of polysaccharides from pericarp tissues of litchi (Litchi chinensis Sonn.) fruit in relation to their antioxidant activities. Carbohydr Res 341:634–638. doi:10.1016/j.carres.2006.01.004
Zhang J, Ma PX (2013) Cyclodextrin-based supramolecular systems for drug delivery: recent progress and future perspective. Adv Drug Deliv Rev 65:1215–1233. doi:10.1016/j.addr.2013.05.001
Acknowledgements
This work is supported by a grant of CNCS-Romania (PN-II-ID-PCE-2011-3-0328). G. I. gratefully acknowledges the sponsorship of the COST Action CM1201. The Super Fluid Extraction System was purchased through a grant of the Romanian National Authority for Scientific Research, CNDI-UEFISCDI, project number 104/2012 (PN-II-PT-PCCA-2011-3.1). The authors are grateful to Global Research SRL, Pitesti for granting access to the microwave extraction oven NEOS, Milestone. The authors declare no conflict of interests.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Neacsu, M.V., Ionita, G., Topala, C. et al. Poly(ethylene glycol)/β-cyclodextrin covalent gel networks: host matrices for studying radical processes in plant extract–riboflavin systems following UV irradiation. Chem. Pap. 71, 607–616 (2017). https://doi.org/10.1007/s11696-016-0047-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-016-0047-x