Abstract
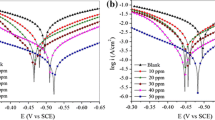
In this study, the corrosion inhibition effectiveness of eight amines, i.e. 2-ethylhexyl amine, aniline, benzylamine, butylamine, ethylamine, isopropylamine, octylamine, and triethanolamine, on C15 grade mild steel in 3 wt% NaCl solution is reported. The corrosion inhibition performance of the amines was studied using immersion tests at 25 and 70 °C, with and without the addition of KI as a possible intensifier. Among the inhibitors tested at 0.1 wt% concentration, the lowest corrosion rates were obtained for specimens immersed in solutions containing 2-ethylhexyl amine at 25 °C and triethanolamine at 70 °C. The highest inhibition effectiveness at 25 °C among all amines tested was obtained for 1.0 wt% butylamine with the addition of 0.5 wt% KI, while at 70 °C the lowest corrosion rate was obtained for specimens inhibited with 1.0 wt% isopropylamine. Surface analysis was subsequently performed on specimens inhibited by the most effective inhibitors. Adsorption of the selected amines on the C15 grade mild steel surface was confirmed by ATR-FTIR. 3D-profilometry showed a reduction in the surface roughness (less corroded) for the specimens inhibited with these inhibitors compared to the non-inhibited specimens. Contact angle measurements showed that all of the tested specimens were hydrophilic.





Similar content being viewed by others
References
Abouchane M, El Bakri M, Touir R, Rochdi A, Elkhattabi O, Ebn Touhami M, Forssal B, Mernari B (2015) Corrosion inhibition and adsorption behavior of triazoles derivatives on mild steel in 1 M H3PO4 and synergistic effect of iodide ions. Res Chem Intermed 41(4):1907–1923. doi:10.1007/s11164-013-1319-5
Adardour K, Touir R, El Bakri M, Larhzil H, Ebn Touhami M, Ramli Y, Zarrouk A, El Kafsaoui H, Essassi EM (2015) Thermodynamic properties and comparative studies of quinoxaline derivatives as a corrosion inhibitor for mild steel in 1 M H2SO4. Res Chem Intermed 41(3):1571–1589. doi:10.1007/s11164-013-1293-y
Ahmad Z (2006) Principles of corrosion engineering and corrosion control. Elsevier, Oxford, UK
Ashassi-Sorkhabi H, Nabavi-Amri SA (2002) Polarization and impedance methods in corrosion inhibition study of carbon steel by amines in petroleum–water mixtures. Electrochim Acta 47(13–14):2239–2244. doi:10.1016/S0013-4686(02)00062-2
Bastidas JM, Damborenea JD, Va´zquez AJ (1997) Butyl substituents in n-butylamine and their influence on mild steel corrosion inhibition in hydrochloric acid. J Appl Electrochem 27(3):345–349. doi:10.1023/a:1018441032374
Bastidas JM, Polo JL, Cano E, Torres CL (2000) Tributylamine as corrosion inhibitor for mild steel in hydrochloric acid. J Mater Sci 35(11):2637–2642. doi:10.1023/a:1004773903987
Chandrasekaran V, Kannan K, Natesan M (2005) Inhibiting properties of some amines on corrosion behaviour of mild steel in phosphoric acid solution at various temperatures. Asian J Chem 17(3):1921–1934
Coates J (2006) Interpretation of infrared spectra, a practical approach. Encyclopedia of analytical chemistry. Wiley, Hoboken, New Jersey, USA
Cymerman CJ, Purushothaman KK (1970) Improved preparation of tertiary amine N-oxides. J Org Chem 35(5):1721–1722. doi:10.1021/jo00830a121
de Damborenea J, Bastidas JM, Vázquez AJ (1997) Adsorption and inhibitive properties of four primary aliphatic amines on mild steel in 2 M hydrochloric acid. Electrochim Acta 42(3):455–459. doi:10.1016/S0013-4686(96)00250-2
Finšgar M (2013) 2-Mercaptobenzimidazole as a copper corrosion inhibitor: part I. Long-term immersion, 3D-profilometry, and electrochemistry. Corros Sci 72:82–89. doi:10.1016/j.corsci.2013.03.011
Finšgar M, Jackson J (2014) Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: a review. Corros Sci 86:17–41. doi:10.1016/j.corsci.2014.04.044
Finšgar M, Kek Merl D (2014) 2-Mercaptobenzoxazole as a copper corrosion inhibitor in chloride solution: electrochemistry, 3D-profilometry, and XPS surface analysis. Corros Sci 80:82–95. doi:10.1016/j.corsci.2013.11.022
Finšgar M, Milošev I (2010) Corrosion behaviour of stainless steels in aqueous solutions of methanesulfonic acid. Corros Sci 52:2430–2438. doi:10.1016/j.corsci.2010.04.001
Fouda AS, Mostafa HA, El-Taib F, Elewady GY (2005) Synergistic influence of iodide ions on the inhibition of corrosion of C-steel in sulphuric acid by some aliphatic amines. Corros Sci 47(8):1988–2004. doi:10.1016/j.corsci.2004.09.008
Fouda AS, Mostafa HA, Elewady GY, El-Hashemy MA (2008) Low molecular weight straight-chain diamines as corrosion inhibitors for SS type 304 in HCl solution. Chem Eng Commun 195(8):934–947. doi:10.1080/00986440801905148
Gao G, Liang C, Wang H (2007) Synthesis of tertiary amines and their inhibitive performance on carbon steel corrosion. Corros Sci 49(4):1833–1846. doi:10.1016/j.corsci.2006.08.014
Ghanbari A, Attar MM, Mahdavian M (2010) Corrosion inhibition performance of three imidazole derivatives on mild steel in 1 M phosphoric acid. Mater Chem Phys 124(2–3):1205–1209. doi:10.1016/j.matchemphys.2010.08.058
Hosseini SMA, Salari M, Jamalizadeh E, Jafari AH (2012) Electrochemical and quantum chemical studies of aromatic amines on the steel corrosion in acid solution. Corrosion 68(7):600–609. doi:10.5006/0494
Massart DL, Vandeginste BGM, Buydens LMC, Jong SD, Lewi PJ, Smeyers-Verbeke J (1997) Handbook of chemometrics and qualimetrics: Part A. Elsevir, Amsterdam
Migahed MA, Al-Sabagh AM, Khamis EA, Zaki EG (2015) Quantum chemical calculations, synthesis and corrosion inhibition efficiency of ethoxylated-[2-(2-{2-[2-(2-benzenesulfonylamino-ethylamino)-ethylamino]-ethylamino}-ethylamino)-ethyl]-4-alkyl-benzenesulfonamide on API X65 steel surface under H2S environment. J Mol Liq 212:360–371. doi:10.1016/j.molliq.2015.09.032
Qian JH, Zhang Y, Yin XY, Liu L (2013) The corrosion inhibitory property of N, N-bis(2-benzimidazolylmethyl)amine for Q235 steel. Mater Corros 64(5):422–425. doi:10.1002/maco.201106300
Sığırcık G, Tüken T, Erbil M (2016) Assessment of the inhibition efficiency of 3,4-diaminobenzonitrile against the corrosion of steel. Corros Sci 102:437–445. doi:10.1016/j.corsci.2015.10.036
Socrates G (2009) Infrared and Raman characteristic group frequencies: tables and charts. Wiley, Chichester
Stewart JE (1959) Vibrational spectra of primary and secondary aliphatic amines. J Chem Phys 30(5):1259–1265. doi:10.1063/1.1730168
Umoren SA, Solomon MM (2015) Effect of halide ions on the corrosion inhibition efficiency of different organic species—a review. J Ind Eng Chem 21:81–100. doi:10.1016/j.jiec.2014.09.033
Zhang HH, Pang X, Zhou M, Liu C, Wei L, Gao K (2015) The behavior of pre-corrosion effect on the performance of imidazoline-based inhibitor in 3 wt% NaCl solution saturated with CO2. Appl Surf Sci 356:63–72. doi:10.1016/j.apsusc.2015.08.003
Zhang D, Tang Y, Qi S, Dong D, Cang H, Lu G (2016) The inhibition performance of long-chain alkyl-substituted benzimidazole derivatives for corrosion of mild steel in HCl. Corros Sci 102:517–522. doi:10.1016/j.corsci.2015.10.002
Acknowledgements
This work was supported by the Slovene Research Agency (Grant No. Z1-6737).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xhanari, K., Grah, N., Finšgar, M. et al. Corrosion inhibition and surface analysis of amines on mild steel in chloride medium. Chem. Pap. 71, 81–89 (2017). https://doi.org/10.1007/s11696-016-0046-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-016-0046-y