Abstract
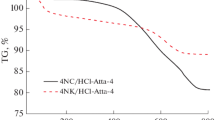
Acid–base bi-functional hydrotalcite like compounds based on partial incorporation of Al3+ into brucite structure of Mg(OH)2 with various molar ratios were prepared through co-precipitation method. The co-precipitation of the precursors produced precipitations followed by drying at 120 °C for 12 h and calcination in air flow at 500 °C for 6 h to obtain the catalysts (Mg–Al HLCs). Many techniques including XRD, TG–DTA, EDX, NH3-TPD, CO2-TPD, GC–MS and XANES were used to characterize and optimize Mg/Al molar ratio based on the thermal stability of the Mg–Al HLCs and their activities in decarboxylation process of coconut oil. The results showed that the best molar ratio of Mg/Al was 3/1 providing a stable hydrotalcite like structure, and the catalyst possessed both acid and base sites on its surface enhancing its activity and selectivity in the decarboxylation process. The catalysts revealed high performance in the decarboxylation process of coconut oil established at 400 °C for 4 h for green hydrocarbons belonging to kerosene fractions.









Similar content being viewed by others
References
Brevoord E, et al (2012) A process for the decarboxylation of fatty acids. European Patent EP 1 996 536 B1
Cavani F, Trifirb F, Vaccari A (1991) Hydrotalcite-type anionic clays: preparation, properties and applications. Catal Today 11:173–301
Constantino VRL, Pinnavaia TJ (1995) Basic properties of Mg 2+1−x Al 3+x layered double hydroxides intercalated by carbonate, hydroxide, chloride, and sulfate anions. Inorg Chem 34(4):883–892
Corma A, Fornes V, Rey F (1994) Hydrotalcites as base catalysts: influence of the chemical composition and synthesis conditions on the dehydrogenation of isopropanol. J Catal 148(1):205–212
Corma A, Martin-Aranda RM (1993) Application of solid base catalysts in the preparation of prepolymers by condensation of ketones and malononitrile. Appl Catal A 105(2):271–279
Di Cosimo JI, Diez VK, Xu M, Iglesia E, Apesteguia CR (1998) Structure and surface and catalytic properties of Mg–Al basic oxides. J Catal 178:499–510
Doesburg EBM, Hoppener RH, de Koning B, Xiaoding X, Scholten JJF, Delmon B, Grange P, Jacobs PA, Poncelet G (1987) Preparation of Catalysts ZV. Elsevier, Amsterdam, p 767
Gastuche MC, Brown G, Mortland MM (1967) Mixed magnesium–aluminium hydroxides. Clay Miner 7:177–192
Mascolo G, Marino O, Miner M (1980) A new synthesis and characterization of magnesium-aluminium hydroxides. Miner Mag 43:619
Mokhtar M, Saleh TS, Basahel SN (2012) Mg–Al hydrotalcites as efficient catalysts for aza-Michael addition reaction: a green protocol. J Mol Catal A Chem 353–354:122–131
Na JG, Han JK, Oh YK, Park JH, Jung TS, Han SS, Yoon HC, Chung SH, Kim JN, Ko CH (2012) Decarboxylation of microalgal oil without hydrogen into hydrocarbon for the production of transportation fuel. Catal Today 185:313–317
Na JG, Yi BE, Kim JN, Yi KB, Park SY, Park JH, Kim JN, Ko CH (2010) Hydrocarbon production from decarboxylation of fatty acid without hydrogen. Catal Today 156:44–48
Pausch I, Lohse HH, Schtirmann K, Alhnann R (1986) Clays Clay Miner 34: 507
Reichle WT (1980) Pulse microreactor examination of the vapor-phase aldol condensation of acetone. J Catal 63(2):295–306
Roelofs JCAA (1974) Activated hydrotalcites as solid base catalysts in aldol condensations, ISBN 90-393-2901-X. Wageningen, Drukkerij Pons en Looijen
Salomoa R, Milena LM, Wakamatsu MH, Pandolfelli VC (2011) Hydrotalcite synthesis via co-precipitation reactions using MgO and Al(OH)3 precursors. Ceram Int 37:3063–3070
Sheldon RA, Dakka J (1994) Heterogeneous catalytic oxidations in the manufacture of fine chemicals. Catal Today 19:215
Tantirungrotechai J, Chotmongkolsap P, Pohmakotr M (2010) Synthesis, characterization, and activity in transesterification of mesoporous Mg–Al mixed-metal oxides. Microporous Mesoporous Mater 128:41–47
Acknowledgements
This work was financially supported by the National Foundation for Science and Technology Development, Vietnam (NAFOSTED) under Grant No. 104.05-2013.57.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nguyen, H.K.D., Nguyen, T.D., Vu, A.T. et al. Preparation of acid–base bi-functional hydrotalcite based catalyst for converting Vietnamese coconut oil to green hydrocarbons. Chem. Pap. 71, 961–970 (2017). https://doi.org/10.1007/s11696-016-0020-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-016-0020-8