Abstract
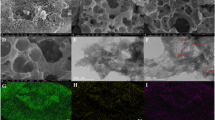
A lanthanum metal–organic framework, [La(BTC)(H2O)(DMF)] (H3BTC = 1, 3, 5-benzenetricarboxylic acid), was synthesized under mild hydrothermal conditions. The synthesized [La(BTC)(H2O)(DMF)] was characterized by scanning electron microscopy in combination with energy dispersive X-ray spectroscopy (SEM/EDS), transmission electron microscopy (TEM), X-ray diffraction (XRD), and fourier transform infrared spectroscopy (FT-IR). Its electrochemical properties and electrocatalytic activity towards H2O2 reduction in acidic media were studied by cyclic voltammetry (CV) and amperometric current–time response. The [La(BTC)(H2O)(DMF)] modified electrode shows good electrochemical behavior and performs well electrocatalytic activity towards hydrogen peroxide (H2O2) reduction at ca. −0.7 V. The modified electrode displays a linear range from 5 μM to 2.67 mM and a limit of detection of 0.73 μM to H2O2. The [La(BTC)(H2O)(DMF)] modified electrode also possesses good selectivity and stability. Thus, [La(BTC)(H2O)(DMF)] will be a promising material for non-enzymatic H2O2 sensor.









Similar content being viewed by others
References
Bashkova S, Bandosz TJ (2013) Insight into the role of the oxidized graphite precursor on the properties of copper-based MOF/graphite oxide composites. Microporous Mesoporous Mater 179:205–211. doi:10.1016/j.micromeso.2013.06.002
Bella F, Bongiovanni R, Kumar RS, Kulandainathan MA, Stephan AM (2013) Light cured networks containing metal organic frameworks as efficient and durable polymer electrolytes for dye-sensitized solar cells. J Mater Chem A 1:9033–9036. doi:10.1039/C3TA12135F
DeYulia GJ, Cárcamo JM, Bórquez Ojeda O, Shelton CC, Golde DW (2005) Hydrogen peroxide generated extracellularly by receptor-ligand interaction facilitates cell signaling. Proc Nat Acad Sci 102:5044–5049. doi:10.1073/pnas.0501154102
Díaz R, Orcajo MG, Botas JA, Calleja G, Palma J (2012) Co8-MOF-5 as electrode for supercapacitors. Mater Lett 68:126–128. doi:10.1016/j.matlet.2011.10.046
Doménech-Carbó A, Doménech-Carbó MT (2008) In situ AFM study of proton-assisted electrochemical oxidation/reduction of microparticles of organic dyes. Electrochem Commun 10:1238–1241. doi:10.1016/j.elecom.2008.06.013
Férey G (2008) Hybrid porous solids: past, present, future. Chem Soc Rev 37:191–214. doi:10.1039/B618320B
Garjonyte R, Malinauskas A (1999) Operational stability of amperometric hydrogen peroxide sensors, based on ferrous and copper hexacyanoferrates. Sens Actuators B Chem 56:93–97. doi:10.1016/S0925-4005(99)00161-6
Guo XD, Zhu GS, Li ZY, Sun FX, Yang ZH, Qiu SL (2006) A lanthanide metal-organic framework with high thermal stability and available Lewis-acid metal sites. Chem Commun 30:3172–3174. doi:10.1039/B605428E
He L, Liu Y, Liu J, Xiong Y, Zheng J, Liu Y, Tang Z (2013) Core–shell noble-metal@metal-organic-framework nanoparticles with highly selective sensing property. Angew Chem Int Ed 52:3741–3745. doi:10.1002/anie.201209903
Hosseini H, Ahmar H, Dehghani A, Bagheri A, Fakhari AR, Amini MM (2013) Au-SH-SiO2 nanoparticles supported on metal-organic framework (Au-SH-SiO2@Cu-MOF) as a sensor for electrocatalytic oxidation and determination of hydrazine. Electrochim Acta 88:301–309. doi:10.1016/j.electacta.2012.10.064
Jahan M, Bao QL, Loh KP (2012) Electrocatalytically active graphene–porphyrin MOF composite for oxygen reduction reaction. J Am Chem Soc 134:6707–6713. doi:10.1021/ja211433h
Kong LR, Lu XF, Bian XJ, Zhang WJ, Wang C (2010) Accurately tuning the dispersity and size of palladium particles on carbon spheres and using carbon spheres/palladium composite as support for polyaniline in H2O2 electrochemical sensing. Langmuir 26:5985–5990. doi:10.1021/la904509v
Kriz K, Kraft L, Krook M, Kriz D (2002) Amperometric determination of l-Lactate based on entrapment of lactate oxidase on a transducer surface with a semi-permeable membrane using a sire technology based biosensor. Application: tomato paste and baby food. J Agric Food Chem 50:419–3424. doi:10.1021/jf0114942
Li HL, Eddaoudi M, O’Keeffe M, Yaghi OM (1999) Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 402:276–279. doi:10.1038/46248
Li JR, Kuppler R, Zhou HC (2009) Selective gas adsorption and separation in metal–organic frameworks. Chem Soc Rev 38:1477–1504. doi:10.1039/B802426J
Li YZ, Huangfu C, Du HJ, Liu WB, Li YW, Ye JS (2013) Electrochemical behavior of metal–organic framework MIL-101 modified carbon paste electrode: an excellent candidate for electroanalysis. J Electro Chem 709:65–69. doi:10.1016/j.jelechem.2013.09.017
Liu SQ, Tang ZY (2010) Nanoparticle assemblies for biological and chemical sensing. J Mater Chem 20:24–35. doi:10.1039/B911328M
Liu HL, Liu YL, Li YW, Tang ZY, Jiang HF (2010) Metal-organic framework supported gold nanoparticles as a highly active heterogeneous catalyst for aerobic oxidation of alcohols. J Phys Chem C 114:13362–13369. doi:10.1021/jp105666f
Lobnik A, Čajlaković M (2001) Sol–gel based optical sensor for continuous determination of dissolved hydrogen peroxide. Sens Actuators B Chem 74:194–199. doi:10.1016/S0925-4005(00)00733-4
Lu G, Hupp JT (2010) Metal-organic frameworks as sensors: a ZIF-8 based Fabry–Pérot device as a selective sensor for chemical vapors and gases. J Am Chem Soc 132:7832–7833. doi:10.1021/ja101415b
Lu HT, Yu S, Fan Y, Yang CP, Xu DL (2013) Nonenzymatic hydrogen peroxide electrochemical sensor based on carbon-coated SnO2 supported Pt nanoparticles. Colloid Surf B 101:106–110. doi:10.1016/j.colsurfb.2012.05.033
Łyszczek R (2007) Thermal and spectroscopic investigations of new lanthanide complexes with 1, 2, 4-benzenetricarboxylic acid. J Therm Anal Calorim 90:533–539. doi:10.1007/s10973-006-7734-8
Mancini MC, Kairdolf BA, Smith AM, Nie SM (2008) Oxidative quenching and degradation of polymer-encapsulated quantum dots: new insights into the long-term fate and toxicity of nanocrystals in vivo. J Am Chem Soc 130:10836–10837. doi:10.1021/ja8040477
Mao JJ, Yang LF, Yu P, Wei XW, Mao LQ (2012) Electrocatalytic four-electron reduction of oxygen with Copper (II)-based metal–organic frameworks. Electrochem Commun 19:29–31. doi:10.1016/j.elecom.2012.02.025
Min KS, Suh MP (2000) Silver (I)-polynitrile network solids for anion exchange: anion-induced transformation of supramolecular structure in the crystalline state. J Am Chem Soc 122:6834–6840. doi:10.1021/ja000642m
Nossol E, Zarbin AJ (2009) A simple and innovative route to prepare a novel carbon nanotube/prussian blue electrode and its utilization as a highly sensitive H2O2 amperometric sensor. Adv Funct Mater 19:3980–3986. doi:10.1002/adfm.200901478
Palanisamy S, Chen S-M, Sarawathi R (2012) A novel nonenzymatic hydrogen peroxide sensor based on reduced graphene oxide/ZnO composite modified electrode. Sens Actuators B Chem 166:372–377. doi:10.1016/j.snb.2012.02.075
Rowsell JL, Yaghi OM (2004) Metal-organic frameworks: a new class of porous materials. Micropor Mesopor Mat 73:3–14. doi:10.1016/j.micromeso.2004.03.034
Santhosh P, Manesh KM, Gopalan A, Lee KP (2006) Fabrication of a new polyaniline grafted multi-wall carbon nanotube modified electrode and its application for electrochemical detection of hydrogen peroxide. Anal Chim Acta 575:32–38. doi:10.1016/j.aca.2006.05.075
Song MJ, Hwang SW, Whang D (2010) Non-enzymatic electrochemical CuO nanoflowers sensor for hydrogen peroxide detection. Talanta 80:1648–1652. doi:10.1016/j.talanta.2009.09.061
Wang MQ, Zhang Y, Bao SJ, Yu YN, Ye C (2016) Ni(II)-based metal-organic framework anchored on carbon nanotubes for highly sensitive non-enzymatic hydrogen peroxide sensing. Electrochem Acta 190:365–370. doi:10.1016/j.electacta.2015.12.199
Welch CM, Banks CE, Simm AO, Compton RG (2005) Silver nanoparticle assemblies supported on glassy-carbon electrodes for the electro-analytical detection of hydrogen peroxide. Anal Bioanal Chem 382:12–21. doi:10.1007/s00216-005-3205-5
Wen YH, Cheng JK, Feng YL, Zhang J, Li ZJ, Yao YG (2005) Synthesis and crystal structure of [La (BTC)(H2O)6]n. Chin J Struct Chem 24:1440–1444
Woo S, Kim YR, Chung TD, Piao Y, Kim H (2012) Synthesis of a graphene–carbon nanotube composite and its electrochemical sensing of hydrogen peroxide. Electrochim Acta 59:509–514. doi:10.1016/j.electacta.2011.11.012
Xu F, Sun YJ, Zhang Y, Shi Y, Wen ZW, Li Z (2011) Graphene–Pt nanocomposite for nonenzymatic detection of hydrogen peroxide with enhanced sensitivity. Electrochem Commun 13:1131–1134. doi:10.1016/j.elecom.2011.07.017
Yang WT, Feng J, Zhang HJ (2012) Facile and rapid fabrication of nanostructured lanthanide coordination polymers as selective luminescent probes in aqueous solution. J Mater Chem 22:6819–6823. doi:10.1039/C2JM16344F
Yang LZ, Xu CL, Ye WC, Liu WS (2015a) An electrochemical sensor for H2O2 based on a new Co-metal-organic framework modified electrode. Sens Actuators B Chem 215:489–496. doi:10.1016/j.snb.2015.03.104
Yang J, Zhou B, Yao J, Jiang XQ (2015b) Nanorods of a new metal–biomolecule coordination polymer showing novel bidirectional electrocatalytic activity and excellent performance in electrochemical sensing. Biosens Bioelectron 67:66–72. doi:10.1016/j.bios.2014.06.047
Yao SJ, Xu JH, Wang Y, Chen XX, Xu YX, Hu SS (2006) A highly sensitive hydrogen peroxide amperometric sensor based on MnO2 nanoparticles and dihexadecyl hydrogen phosphate composite film. Anal Chim Acta 557:78–84. doi:10.1016/j.aca.2005.10.052
Zhang JD, Oyama M (2004) A hydrogen peroxide sensor based on the peroxidase activity of hemoglobin immobilized on gold nanoparticles-modified ITO electrode. Electrochim Acta 50:85–90. doi:10.1016/j.electacta.2004.07.026
Zhang YL, Liu YP, He JM, Pang PF, Gao YT, Hu QF (2013) Electrochemical behavior of graphene/Nafion/Azure I/Au nanoparticles composites modified glass carbon electrode and its application as nonenzymatic hydrogen peroxide sensor. Electrochim Acta 90:550–555. doi:10.1016/j.electacta.2012.12.068
Zhang DJ, Zhang JC, Shi HZ, Guo XL, Guo YY, Zhang RC, Yuan BQ (2015a) Redox-active microsized metal–organic framework for efficient nonenzymatic H2O2 sensing. Sens Actuators B Chem 221:224–229. doi:10.1016/j.snb.2015.06.079
Zhang DJ, Zhang JC, Zhang RC, Shi HZ, Guo YY, Guo XL, Li SJ, Yuan BQ (2015b) 3D porous metal–organic framework as an efficient electrocatalyst for nonenzymatic sensing application. Talanta 144:1176–1181. doi:10.1016/j.talanta.2015.07.091
Zhao W, Wang HC, Qin X, Wang XS, Zhao ZX, Miao ZY, Chen LL, Shan MM, Fang YX, Chen Q (2009) A novel nonenzymatic hydrogen peroxide sensor based on multi-wall carbon nanotube/silver nanoparticle nanohybrids modified gold electrode. Talanta 80:1029–1033. doi:10.1016/j.talanta.2009.07.055
Zhao MT, Deng K, He LC, Liu Y, Li GD, Zhao HJ, Tang ZY (2014) Core–shell palladium nanoparticle@metal–organic frameworks as multifunctional catalysts for cascade reactions. Am Chem Soc 136:1738–1741. doi:10.1021/ja411468e
Zhu X, Zheng HY, Wei XF, Lin ZY, Guo LH, Qiu B, Chen G (2013) Metal–organic framework (MOF): a novel sensing platform for biomolecules. Chem Commun 49:1276–1278. doi:10.1039/C2CC36661D
Acknowledgements
This work was supported by the Natural Science Foundation of Fujian Province in China (2016J01067), the Program for New Century Excellent Talents in Minnan Normal University (MX14003), the Project for Visiting Abroad of Fujian Province and Minnan Normal University, and the Innovation Base Foundation for Graduate Students Education of Fujian Province.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Y., Zhong, Y. & Huang, J. The synthesis of a lanthanum metal–organic framework and its sensitivity electrochemical detection of H2O2 . Chem. Pap. 71, 913–920 (2017). https://doi.org/10.1007/s11696-016-0011-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-016-0011-9