Abstract
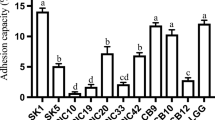
In this study, the probiotic characteristics of Lactobacillus plantarum isolated from an Iranian traditional fermented product, Yellow Zabol Kashk (YZK), were evaluated. The isolated strain was identified as Lactobacillus plantarum TW29-1 by 16S rRNA gene sequencing. L. plantarum TW29-1 had a remarkable tolerance to acidic pHs, bile salts, and simulated gastrointestinal juices. The strain also showed a 51.44% cell surface hydrophobicity, 40.49% auto-aggregation, and 28.63% co-aggregation. L. plantarum TW29-1 was found to have 12.5% adhesion, and it was also able to compete (50.19%) and inhibit (48.87%) Salmonella typhimurium adherence to Caco-2 cells. The growth of pathogenic bacteria (i.e., Pseudomonas aeruginosa, Listeria innocua, Staphylococcus aureus, Escherichia coli, and S. typhimurium) was strongly inhibited by the isolate and S. aureus was the sensitive strain. L. plantarum TW29-1 did not induce DNase or haemolytic activity, confirming its safety aspects. Based on the results, L. plantarum TW29-1 could be introduced as a novel probiotic strain with therapeutic and preservation properties for food and health-promoting purposes.





Similar content being viewed by others
References
S. Rezac, C.R. Kok, M. Heermann, R. Hutkins, Fermented foods as a dietary source of live organisms. Front. Microbiol. 9, 1785 (2018)
A. Peters, P. Krumbholz, E. Jäger, A. Heintz-Buschart, M.V. Çakir, S. Rothemund, A. Gaudl, U. Ceglarek, T. Schöneberg, C. Stäubert, Metabolites of lactic acid bacteria present in fermented foods are highly potent agonists of human hydroxycarboxylic acid receptor 3. PLoS Genet. 15(3), e1008145 (2019)
S.E. Gilliland, Health and nutritional benefits from lactic acid bacteria. FEMS Microbiol. Rev. 7(1–2), 175–188 (1990)
A. Vasiee, F. Falah, B. Alizadeh Behbahani, F. Tabatabaee-Yazdi, Probiotic characterization of Pediococcus strains isolated from Iranian cereal-dairy fermented product: interaction with pathogenic bacteria and the enteric cell line Caco-2. J. Biosci. Bioeng. 130(5), 471–479 (2020)
F. Falah, A. Vasiee, B. Alizadeh Behbahani, F.T. Yazdi, S. Moradi, S.A. Mortazavi, S. Roshanak, Evaluation of adherence and anti-infective properties of probiotic Lactobacillus fermentum strain 4–17 against Escherichia coli causing urinary tract infection in humans. Microb. Pathog. 131, 246–253 (2019)
Y.F. Tabatabaei, A. Vasiee, B.B. Alizadeh, S. Mortazavi, Diversity of lactic acid bacteria isolated from yellow zabol kashk using 16S rRNA gene sequence analysis. Iran. J. Food Sci. Technol. 59(13), 25–36 (2017)
A. Guidone, T. Zotta, R.P. Ross, C. Stanton, M.C. Rea, E. Parente, A. Ricciardi, Functional properties of Lactobacillus plantarum strains: a multivariate screening study. LWT-Food Sci. Technol. 56(1), 69–76 (2014)
S.S. Behera, R.C. Ray, N. Zdolec, Lactobacillus plantarum with functional properties: an approach to increase safety and shelf-life of fermented foods. BioMed. Res. Int. 2018, 9361614 (2018)
E.P.O.B. Hazards, A. Ricci, A. Allende, D. Bolton, M. Chemaly, R. Davies, P.S.F. Escámez, Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 5: suitability of taxonomic units notified to EFSA until September 2016. EFSA J. 15(3), e04663 (2017)
F.F. Jia, L.J. Zhang, X.H. Pang, X.X. Gu, A. Abdelazez, Y. Liang, S.R. Son, X.C. Meng, Complete genome sequence of bacteriocin-producing Lactobacillus plantarum KLDS1.0391, a probiotic strain with gastrointestinal tract resistance and adhesion to the intestinal epithelial cells. Genomics 109(5–6), 432–437 (2017)
M. Hojjati, B. Alizadeh Behabahani, F. Falah, Aggregation, adherence, anti-adhesion and antagonistic activity properties relating to surface charge of probiotic Lactobacillus brevis gp104 against Staphylococcus aureus. Microb. Pathog. 147, 104420 (2020)
A.M.O. Leite, M.A.L. Miguel, R.S. Peixoto, P. Ruas-Madiedo, V.M.F. Paschoalin, B. Mayo, S. Delgado, Probiotic potential of selected lactic acid bacteria strains isolated from Brazilian kefir grains. J. Dairy Sci. 98(6), 3622–3632 (2015)
M.E. Fadda, V. Mosa, M. Deplano, M.B. Pisano, S. Cosentino, In vitro screening of Kluyveromyces strains isolated from Fiore Sardo cheese for potential use as probiotics. LWT 75, 100–106 (2017)
E. Kiray, E. Kariptas, S.Y. Azarkan, Evaluation of vaginal lactobacilli with potential probiotic properties and biotherapeutic effects isolated from healthy Turkish women. PSM Microbiol. 4(3), 56–70 (2019)
R. Georgieva, L. Yocheva, L. Tserovska, G. Zhelezova, N. Stefanova, A. Atanasova et al., Antimicrobial activity and antibiotic susceptibility of Lactobacillus and Bifidobacterium spp. intended for use as starter and probiotic cultures. Biotechnol. Biotech. Eq. 29(1), 84–91 (2015)
S. Momenzadeh, H. Jooyandeh, B. Alizadeh Behbahani, H. Barzegar, Evaluation of probiotic and antibacterial properties of Lactobacillus fermentum SL163–4. Iran. J. Food Sci. Technol. Res. 17(2), 233–242 (2021)
J.S. Zhou, C.J. Pillidge, P.K. Gopal, H.S. Gill, Antibiotic susceptibility profiles of new probiotic Lactobacillus and Bifidobacterium strains. Int. J. Food Microbiol. 98(2), 211–217 (2005)
H. Gupta, R.K. Malik, Incidence of virulence in bacteriocin-producing enterococcal isolates. Le Lait. 87(6), 587–601 (2007)
S. Pieniz, R. Andreazza, T. Anghinoni, F. Camargo, A. Brandelli, Probiotic potential, antimicrobial and antioxidant activities of Enterococcus durans strain LAB18s. Food Control 37, 251–256 (2014)
P.D. Cotter, C. Hill, Surviving the acid test: responses of Gram-positive bacteria to low pH. Microbiol. Mol. Boil. Rev. 67(3), 429–453 (2003)
A.R. Madureira, C.I. Pereira, K. Truszkowska, A.M. Gomes, M.E. Pintado, F.X. Malcata, Survival of probiotic bacteria in a whey cheese vector submitted to environmental conditions prevailing in the gastrointestinal tract. Int. Dairy J. 15(6–9), 921–927 (2005)
M. Nooshkam, A. Babazadeh, H. Jooyandeh, Lactulose: properties, techno-functional food applications, and food grade delivery system. Trends Food Sci. Technol. 80, 23–34 (2018)
J. Šušković, B. Kos, S. Matošić, V. Besendorfer, The effect of bile salts on survival and morphology of a potential probiotic strain Lactobacillus acidophilus M92. World. J. Microbiol. Biotechnol. 16(7), 673–678 (2000)
A. Bezkorovainy, Probiotics: determinants of survival and growth in the gut. Am. J. Clin. Nutr. 73(3), 399s–405s (2001)
H. Zhu, C.A. Hart, D. Sales, N.B. Roberts, Bacterial killing in gastric juice–effect of pH and pepsin on Escherichia coli and Helicobacter pylori. J. Med. Microbiol. 55(9), 1265–1270 (2006)
B. Alizadeh Behbahani, M. Noshad, F. Falah, Inhibition of Escherichia coli adhesion to human intestinal Caco-2 cells by probiotic candidate Lactobacillus plantarum strain L15. Microb. Pathog. 136, 103677 (2019)
H. Gandomi, A. Farhangfar, A. Akhondzadeh Basti, A. Misaghi, N. Noori, Auto and co-aggregation, hydrophobicity and adhesion properties of Lactobacillus plantarum strains isolated from Siahmazgi traditional cheese. Food Health 2(1), 1–5 (2019)
S.H. Son, H.L. Jeon, S.J. Yang, N.K. Lee, H.D. Paik, In vitro characterization of Lactobacillus brevis KU15006, an isolate from kimchi, reveals anti-adhesion activity against foodborne pathogens and antidiabetic properties. Microb. Pathog. 112, 135–141 (2017)
K.M.G.M.M. Kariyawasam, S.J. Yang, N.K. Lee, H.D. Paik, Probiotic properties of Lactobacillus brevis KU200019 and synergistic activity with Fructooligosaccharides in antagonistic activity against foodborne pathogens. Food Sci. Anim. Resour. 40(2), 297–310 (2020)
T. Janković, J. Frece, M. Abram, I. Gobin, Aggregation ability of potential probiotic Lactobacillus plantarum strains. Int. J. Sanit. Eng. Res 6(1), 19–24 (2012)
D.N. Furtado, S.D. Todorov, M. Landgraf, M.T. Destro, B.D.G.M. Franco, Bacteriocinogenic Lactococcus lactis subsp: lactis DF04Mi isolated from goat milk: evaluation of the probiotic potential. Braz. J. Microbiol. 45(3), 1047–1054 (2014)
R.K. Duary, Y.S. Rajput, V.K. Batish, S. Grover, Assessing the adhesion of putative indigenous probiotic lactobacilli to human colonic epithelial cells. Indian J. Med. Res. 134(6), 664–671 (2011)
E.M. Tuomola, S.J. Salminen, Adhesion of some probiotic and dairy Lactobacillus strains to Caco-2 cell cultures. Int. J. Food Microbiol. 41(1), 45–51 (1998)
M.A. Bianchi, D. Del Rio, N. Pellegrini, G. Sansebastiano, E. Neviani, F. Brighenti, A fluorescence-based method for the detection of adhesive properties of lactic acid bacteria to Caco-2 cells. Lett. Appl. Microbiol. 39(3), 301–305 (2004)
A. Nowak, I. Motyl, In vitro anti-adherence effect of probiotic Lactobacillus strains on human enteropathogens. Biotechnol. Food Sci. 81, 103–112 (2017)
A.S. Dhanani, S.B. Gaudana, T. Bagchi, The ability of Lactobacillus adhesin EF-Tu to interfere with pathogen adhesion. Eur. Food Res. Technol. 232(5), 777–785 (2011)
A.C. Ouwehand, S. Salminen, In vitro adhesion assays for probiotics and their in vivo relevance: a review. Microb. Ecol. Health Dis. 15(3), 175–184 (2003)
Y.K. Lee, C.Y. Lim, W.L. Teng, A.C. Ouwehand, E.M. Tuomola, S. Salminen, Quantitative approach in the study of adhesion of lactic acid bacteria to intestinal cells and their competition with enterobacteria. Appl. Environ. Microb. 66(9), 3692–3697 (2000)
M.L. Chikindas, R. Weeks, D. Drider, V.A. Chistyakov, L.M. Dicks, Functions and emerging applications of bacteriocins. Curr. Opin. Biotechnol. 49, 23–28 (2018)
C. Altieri, A. Bevilacqua, D. Cardillo, M. Sinigaglia, Effectiveness of fatty acids and their monoglycerides against Gram-negative pathogens. Int. J. Food Sci. Technol. 44(2), 359–366 (2009)
L. Makras, L. De Vuyst, The in vitro inhibition of Gram-negative pathogenic bacteria by bifidobacteria is caused by the production of organic acids. Int. Dairy J. 16(9), 1049–1057 (2006)
E.H. Ryu, H.C. Chang, In vitro study of potentially probiotic lactic acid bacteria strains isolated from kimchi. Ann. Microbiol. 63(4), 1387–1395 (2013)
C.C. Tsai, H.Y. Hsih, H.H. Chiu, Y.Y. Lai, J.H. Liu, B. Yu, H.Y. Tsen, Antagonistic activity against Salmonella infection in vitro and in vivo for two Lactobacillus strains from swine and poultry. Int. J. Food Microbiol. 102(2), 185–194 (2005)
Acknowledgements
The authors would like to express their sincere gratitude to the Vice-chancellor for Research and Technology of Agricultural Sciences and Natural Resources University of Khuzestan for supporting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saboktakin-Rizi, M., Alizadeh Behbahani, B., Hojjati, M. et al. Identification of Lactobacillus plantarum TW29-1 isolated from Iranian fermented cereal-dairy product (Yellow Zabol Kashk): probiotic characteristics, antimicrobial activity and safety evaluation. Food Measure 15, 2615–2624 (2021). https://doi.org/10.1007/s11694-021-00846-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-021-00846-5