Abstract
In this study, a capacitance thermal analyzer (CTA) was designed and tested for measuring the melting properties of chocolates, and compared with those measured by DSC and dynamic rheology. Chocolates with different fat content and particle size distribution (PSD) were placed between stainless steel plates, while capacitance and temperature were monitored between 20 and 60 °C. The PSD did not influence the Tonset (~ 25 °C) and Tpeak (33 °C) measured by DSC. However, samples with finer particles had lower Tend than those with coarser particles (36.59–37.28 °C). Varying fat content did not result in differences in the DSC melting curves. Samples with smaller particle sizes had lower temperatures at peak capacitance than those with larger particles, with peak temperatures ranging from 30.8 to 39.3 °C, while higher peak capacitance values (2.61–2.84 10− 11 F) were measured by CTA. Samples with higher fat content had lower peak temperatures (range 34.7–39.71 °C) but higher peak capacitance values (range 3.29–4.3 10− 11F). Values from the CTA were best correlated with results determined by dynamic thermal rheometry.
Similar content being viewed by others
Introduction
Chocolate is a complex semi-solid suspension of fine particles containing cocoa, sugar, and sometimes dairy ingredients in a continuous fat phase [1]. Chocolates are categorized as dark, white, or milk chocolate based on the content of cocoa mass, dairy ingredients and cocoa butter. The production of chocolate starts from raw cocoa beans, which are then subjected to fermentation, roasting, milling, winnowing, refining, and tempering. The melting properties of chocolates are critical to their quality because they greatly influence consumer acceptability, appearance and storage stability of the product. Improper processing can lead to undesirable melting temperatures and crystal states, along with loss of glossy appearance or formation of fat bloom during storage [2,3,4].
One important factor that influences melting properties is the crystal form of the fat phase in chocolate. The fat crystals may exhibit polymorphism, that is they may have more than two distinct crystalline forms in the same sample [2]. At least six polymorphic forms have been identified, designated Forms I through VI in the Roman numeral system [5]. The forms have different melting temperatures: 16–18 °C for Form I (γ), 22–24 °C for Form II (α), 24–26 °C for Form III (β′), 26–28 °C for Form IV(β1′), 32–34 °C for Form V (β1), and 34–36 °C for form VI (β2). The Greek letter designations refer to the way in which triglycerides are packed into a unit cell in the crystal structure. The preferred polymorph is Form V in most cases. Thus, most chocolate undergoes tempering to ensure that mostly Form V exists in the finished product. Typically, the chocolate is heated just enough (~ 50 °C) to melt and remove all crystal structures from the cocoa butter. The chocolate is cooled to ~ 27 °C allowing Form V, and some Form IV, crystals to grow. The product temperature is then raised (~ 29–31 °C) to melt off Form IV crystals, and Form V seed crystals may be added to encourage the process.
Particle size distribution and fat content of the chocolate are also important factors influencing appearance, consumer acceptance, and processing properties. The influence of these factors on the rheological properties, texture and consumer evaluation of chocolates have been well studied. Afoakwa et al. [6] found that higher fat content and smaller particle size decreased the plastic viscosity of molten dark chocolate. Some researchers have noted that large particle size of the cocoa mass can lead to sensations of grittiness or coarseness in the mouth [7]. Do et al. [8] studied how fat content and particle distribution of chocolate liquor influence the hardness and heat resistance of solid chocolates. They also found that chocolates with higher fat content need longer heating times to be completely melted. In addition, chocolates with finer particles exhibited longer melting times than those with larger particles. However, the influence of fat content and particle size distribution on melting temperatures has been barely studied.
Differential scanning calorimetry (DSC) is one of the most commonly used methods to determine the melting properties of chocolates. The DSC compares the flow of heat between a sample and empty reference pan during a constantly changing temperature. Large changes in heat flow are indicative of increases in specific heat related to phase transitions. Often the heat flow change during a phase transition is not a specific point, but associated with a temperature range. Therefore, there are several ways to characterize melting properties. That is, starch gelatinization, fat melting and other first-order transitions can be characterized by an onset temperature (Tonset), peak temperature (Tpeak), ending temperature (Tend) and enthalpy of melting (∆Hmelt) [9].
The melting properties of chocolates have also been studied by dynamic thermal rheology [10]. These instruments measure the storage modulus (G′) or loss modulus (G″). As cocoa butter melts, the firm chocolate becomes softer and may begin to flow. Thus, G′ is a good indicator to show the beginning and the end of chocolate melting. Generally, G′ is on the order of 103–104 Pa for the solid and diminishes to 100–101 Pa once melted.
Dielectric thermal analysis (DETA) is another method to study phase transitions of food materials. DETA is carried out by a dielectric analyzer, which measures the change of dielectric constant ε′ and dielectric loss ε″ of a food material as a function of temperature or AC frequency. The ε′ is the ability of a material to store energy in response to an applied electric field. In contrast, ε″ describes the ability of one material to dissipate energy in response to an applied electric field, and that typically results in heat generation [11]. The dielectric loss of a food material reaches a peak value when it reaches the second-order transition temperature, as with a glass transition. On the other hand, when a food material undergoes a first-order transition such as melting, the dielectric constant will increase greatly [12].
Capacitance is an electrical property dependent upon the dielectric properties of the medium. However, it is an easier property to measure as it does not require determination of both the real and imaginary components of ε*. In addition, many capacitance measuring instruments determine values at discrete frequencies. Thus, they are much simpler to build and less expensive than full-scale network analyzers. In this study, a capacitance based thermal analysis (CTA) system was proposed to study the melting properties of chocolate. The apparatus was constructed at the University of Georgia using metal plates and an inexpensive capacitance meter. Capacitance is the ability of a subject to store electrical charge, and the capacitance of a parallel-plate capacitor can be determined as:
where ε′ is dielectric constant or permittivity of the medium between the parallel-plates, ε0 is the dielectric constant when the medium is air, \(A\)is the overlap area (completely and exclusively filled with medium) of the two parallel plates, and \(d\) is the distant between the two plates. The value of \({{\upvarepsilon }}_{0}\) varies only by 0.005% as temperature increases from 0 to 50 °C [13]. A medium such as food, particularly when undergoing a phase transition, will have an ε′ and capacitance that changes with temperature.
A few studies have used capacitance sensors similar to the CTA to study the glass transition and melting temperatures of frozen carbohydrate and NaCl solutions [14]. They found transition temperatures similar to those measured by differential scanning calorimetry. A similar system has been successful for measuring glass transitions in soft and hard sugar candies [15]. In this study, a system is described to study the melting properties of chocolates with different fat content, particle size distribution and cocoa mass content. DSC and viscoelastic properties were also measured for comparison.
Frozen or glassy aqueous systems containing salts or low molecular weight compounds such as sugars and acids undergo a large change in ε′ when transitioning from solid to liquid. In contrast, high fat systems have comparatively smaller differences in ε′ between solid and liquid states. Thus, the cell was modified from previous versions to enhance the measured differences between states. The modified CTA consists of one smaller upper plate and one larger base plate. During a temperature scan, the chocolate sample (with larger area than the upper plate but smaller than the base plate) melts and collapses when the temperature reaches its melting point. The composition of the medium between the parallel plates changes from chocolate only to layers of chocolate and air. If the layers are separate, the capacitance of the medium is given by:
where \(\theta \left(T\right)\) is the volume fraction of chocolate in the medium at a specific temperature.
The objective of this research was to evaluate the use of a modified CTA system to measure melting transitions in chocolate. These were compared to results measured by DSC and rheometry. In addition, samples with differing cocoa mass particle size and fat content were tested to determine if these had an influence on the measured properties.
Materials and methods
Materials
Cocoa mass containing 70.4% cocoa solids was purchased from Callebaut (Wieze, Belgium). Raw organic cocoa butter was purchased from Saaqin (Hicksville, New York). Raw cocoa nibs were provided by Cocoatown (Roswell, Atlanta). Commercial chocolates were purchased from a Kroger grocery in Athens, Georgia.
Preparation of chocolate samples
Chocolate samples with different fat content were produced by mixing raw cocoa butter with cocoa mass in percentages of 10, 20, 30, 40, and 50% by weight. Each mix was treated in a tempering machine (Model Revolution 2, ChocoVision Corp., Poughkeepsie, NY). The program heating schedule included an increase to 46 °C (over 10 min) followed by a decrease to 27 °C (over 12 min). At that point Form V chocolate seed crystals were added followed by an increase in temperature to 31 °C with a hold for 5 min. The well-tempered chocolate was poured into to 5 cm × 5 cm × 1.5 cm chocolate molds (Wilton INC. Woodridge, IL) and held overnight at 4 °C .
Particle size of the chocolate samples was controlled by varying the grinding time. Raw cocoa butter was mixed with cocoa nibs in a percentage of 10% and processed by a double-conical stone grinding concher (Model ECGC05, Cocoatown, Roswell, Atlanta). About 5 ml cocoa liquid was taken from the mix for particle size analysis every 15 min for the first 2 h, and every 1 h thereafter. Five different batches of cocoa butter and mass were processed in the concher for 15, 30 min, 1 h, 2, 4, and 12 h, respectively. The final chocolate liquor from each batch was used to make chocolate samples using the tempering method described above.
Particle size distribution
The particle size distribution was determined by laser diffraction using a Malvern Mastersizer (Model MSS, Malvern Instruments Ltd., Malvern, England). The presentation was selected as “Custom” with refractive indices of \({\text{n}}_{{({\text{CH}}_{3} )_{2} {\text{CHOH}}}}\)n = 1.378 and nparticle = 1.590 [16]. About 2 g of chocolate sample was melted and dispersed with 30 ml isopropanol in a 50 ml centrifuge tube, then mixed with a vortex mixer for 2 min. The samples were then introduced into the Mastersizer dispersion mixer until an obscuration of 20% was reached. Between samples, the detector and laser were aligned and backgrounds were calibrated. The size distribution was expressed as the relative volume of particles in each size range (Malvern MasterSizer Micro Software v 2.19). Several particle size distribution parameters were determined including d10, d50 and d90, that is the particle size below which 10, 50 or 90% of the sample lies. In addition, the Sauter mean diameter (d3,2) and volume mean diameter (d4,3) were determined.
Capacitance based thermal analysis (CTA)
The parallel-plate capacitor cell (Fig. 1) was built at the University of Georgia Instrument Design and Fabrication Shop (Athens, GA). It consisted of a 100 mm diameter stainless base plate, a 60 mm diameter stainless upper plate, a cylindrical Teflon side wall, and a threaded Teflon cap through which a 12.5 mm diameter stainless screw could turn, and which was welded to the upper plate. This allowed the upper plate to be spaced a specified distance from the base plate. The base plate and upper knob had 4 mm holes drilled to provide connection with a banana or needle plug. A cylinder spacer was made from Teflon with outer diameter 100 mm, inner diameter 80 and 100 mm in height. The lower side was fixed to the base plate by a groove on the base plate.
Each chocolate sample was placed on the base plate and the upper plate lowered until it contacted the sample. The base plate was then heated by a Thermolyne Nuova hotplate at constant temperature (80 °C). The measurement of capacitance was done with an LCR meter (NI PXI 4072, National Instrument Corporation, Austin, TX) attached to a PXI chassis (NI PXI 1042, National Instrument Corporation, Austin, TX). A T-type thermocouple (Probe 1/16′′ diameter, OMEGA Engineering, Stamford, CT) penetrated the base plate to just make contact with the sample on one side. Temperature data was collected with a data acquisition board (NI 9219, National Instrument Corporation, Austin, TX). The virtual instrument interface and data collection were accomplished with LabVIEW software (Version 2015, National Instrument Corporation, Austin, TX).
DSC
The melting temperature of each sample was determined by differential scanning calorimetery (Model DSC 1, Mettler-Toledo International Inc., Greifensee, Switzerland). Approximately 12 mg of each sample was sealed into a 40 μl aluminum pan. An empty pan was used as a reference sample. Dry nitrogen gas was used to minimize water condensation in the measuring cell. The temperature was scanned from 0 to 60 °C at a heating rate of 5 °C per minute. The onset temperature (Tonset), peak temperature (Tpeak), end temperature (Tend) and enthalpy of melting (∆Hmelt) were calculated using the STARe Thermal Analysis Software. The melting index (Tindex) was computed as (Tend − Tonset). Each sample was analyzed in triplicate and mean values and standard deviations reported.
Dynamic rheological analyses
The viscoelastic properties of the samples as a function of temperature were measured with a dynamic rheometer (Model Discovery HR-2, TA Instrument Inc., New Castle DE). Before the tests were initiated, the samples were melted and tempered in the rheometer. Approximately 4 g of each chocolate sample was placed on the temperature-controlled stage below a 40 mm cone and plate probe (1.997°), then raised to 48 °C at a rate of 5 °C/min. The sample was then cooled to 26.7 °C at a rate of 2 °C/min. After a 5 min rest period, the temperature was raised to 31.6 °C at a rate of 2 °C/min. After the solid chocolate was formed, the sample was then cooled to 10 °C at a rate of 15 °C/min. The instrument was run in the small-strain oscillatory mode, with dynamic strains set to 1%. The temperature ramp was set at 10–60 °C at a rate of 5 °C/min.
Statistical methods
All tests were repeated at least three times and results presented as the mean and standard deviation. The results were compared by one-way ANOVA using SAS 9.3 (SAS Institute Inc., Cary NC) to determine the effects of the fat content and the particle size distribution of the chocolate sample on the melting properties measured by DSC and CTA. Tukey′s HSD was used to determine significant differences amongst treatments at the 95% level of confidence.
Results and discussion
Particle size reduction and particle size distribution
The largest particle size (d90) for a typical cocoa mix (fat and nibs) is shown in Fig. 2 as a function of grinding time. A compilation of the size and distribution measurements (d10, d50, d90, d32, d4,3) is shown in Table 1. Most of the particle size reduction was achieved within the first 2 h of grinding. The initial d90 was 77 ± 22 µm, which was reduced to 15.5 ± 5.3 µm within 4 h, to 11.7 ± 4.1 µm within 8 h, and finally reached a constant value of ~ 8 µm (Fig. 2). As shown in Table 1, the d90 and d4,3 were significantly smaller after 15 min, 30 min, 4 h and 8 h. The smallest diameter particle (d10), however, did not decrease after 15 min. This indicates that the stone grinding is most effective at reducing the size of large particles, but a point is reached at which no further reduction in particle size is achieved.
A few researchers have studied the effects of particle size on the properties of chocolate products. Bolenz and Manske [17] researched the impact of fat content during grinding on particle size distribution in chocolate milk. Afoakwa et al. [18] reviewed the role that particle size distribution and ingredients have in determining rheological and sensory properties of dark chocolate. In these cases, the researchers used a three-roller refiner followed by sifting to control particle size distribution, thus quickly creating fairly uniform particle size distributions with one peak. In our case, particle properties were determined solely by the grinding time. This is more reflective of industry practices, where stone grinding is often used to create small and uniform particle sizes after long grinding times.
Figure 3 shows how the particle size distribution changed during refining. Initially, there is a trimodal distribution with sizes centered around 1, 8 and 190 µm. During grinding, the peak with largest particles diminished, and disappeared within 4 h. In conjunction, the middle peak shifted to smaller sizes, with the peak value going from 9 to 5 µm. The volume fraction of the medium size particles also increased with time, suggesting that this fraction grew at the expense of the larger particles.
DSC measurements of chocolate melting
The melting temperatures of chocolate samples measured by DSC are shown as a function of the refining time (Table 2) or fat content (Table 3). In general, particle size did not greatly affect the melting temperature as measured be either Tonset and Tpeak. Samples with smaller particles had slightly higher Tonset and slightly lower Tend (and therefore smaller Tindex).
The differences were small, however, with Tend ranging from 36.59 ± 0.17 to 37.28 ± 0.31 °C and Tindex from 11.13 ± 0.22 to 12.68 ± 0.19 °C. Other researchers have seen similar trends [2, 19], suggesting that in chocolate, particle size distribution (PSD) has only a small influence on melting properties. They concluded that chocolates with finer particles would take longer time to melt than their corresponding products with larger particles. There is no particular reason that the lipid phase would have differing properties due to the cocoa mass particles. Thus, any measured differences in melting behavior might be attributed to different crystal sizes or polymorphs, or more likely due to differences in heat transfer rates in samples with different size particles.
Differences in fat content (10–50%) resulted in slightly different Tonset, Tpeak and Tend values. Tonset varied from 25.70 ± 0.14 to 26.11 ± 0.23 °C with increasing fat content, while Tend varied from 36.33 ± 0.25 to 37.47 ± 0.31 °C. Thus, Tindex (reflective of peak width) increased from 10.22 ± 0.07 to 11.77 ± 0.16 °C. Again, this may reflect differences of heat transfer rates in the samples. In addition, samples with more fat will take longer to melt, and this plays out even as the calorimeter temperature is constantly increasing. Previous studies have shown that the melting properties can be influence by PSD, crystallization form [20, 21], lipid types, additives, and composition [22] of chocolates. Others have suggested that the amount of fat in dark chocolates influences the degree of crystallinity and crystal size distribution (CSD) of the samples [23].
Dynamic rheological analyses of chocolate melting
Changes in the dynamic rheological properties during temperature scans of chocolate were best characterized by the storage modulus (G′) (Table 4). In general, G′ in the solid state was ~ 103–104 Pa and decreased over a short temperature range corresponding to melting, to values near 101 Pa in the molten state. The beginning of melting was characterized as Tonset, where the gradient of this temperature point is at least 3 times greater than the previous three data points. The end of melting was characterized as Tend, where the gradient after Tend was less than 10% less than its previous three data points. The Tindex of each sample was calculated as Tend − Tonset.
As found with the DSC results, increasing fat content resulted in larger Tindex values, indicating samples with higher fat content had a broader temperature range over which they melted (Table 5). In addition, the Tonset and Tendset measured by the rheometer were similar to DSC measurements, but did have slightly greater Tonset values. The difference may be attributed to the differing sample sizes (~ 3 g vs. ~ 10 mg) required by the rheometer and DSC, respectively, and that different physical phenomena are being measured by the two instruments.
However, rheometric measurements did show a greater range in Tonset (25.3 ± 0.4 to 28.1 ± 0.3 °C) than measured by DSC, and greater statistical significance amongst values measured for samples with differing fat levels.
CTA measurements of chocolate melting
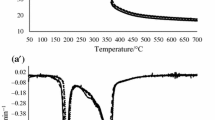
Figure 4 shows a representative figure of how capacitance changes with temperature for chocolate samples in the CTA device. In general, capacitance values were in the picofarad range for samples in the solid state. As the temperature increased there was a slight increase in the capacitance, followed by a decrease after melting had commenced. The initial increase is likely due to an increase in the dielectric constant ε′ as temperature increased, stemming from increased molecular mobility. However, changes would only be slight for molecules in the solid phase. There are different lipid fractions in cocoa butter, and some may begin to melt at lower temperatures. For example, Torbica et al. [24] showed that cocoa butter had a solid fat content of 82, 76, 51 and 8% at temperatures of 20, 25, 30 and 35 °C, respectively.
At a critical temperature, the capacitance began to drop. This coincided with melting of the chocolate, and its flow into the outer chamber, which began to reduce the volume of chocolate between the plates. The receding chocolate was replaced by air, which has a much lower dielectric constant (1.00059 at 20 °C), and thus the measured capacitance began to decrease.
The particle size distribution (PSD) of the samples significantly influenced the temperature at peak capacitance, which ranged from 30.8 ± 0.6 to 39.3 ± 0.4 °C (Table 6), while peak capacitance values ranged from 26.1 ± 0.4 to 28.4 ± 0.3 pF. That is, finer PSD resulted in lower peak capacitance temperature and greater peak capacitance value. As previously noted, larger cocoa mass particle size can produce chocolate that is more difficult to melt. This likely resulted in higher peak temperatures for the CTA. In addition, and by design, the heating rate in the CTA was not carefully regulated, and this also resulted in slower melting.
It has also been observed that PSD influences the rheological properties of chocolate. Samples with larger PSD yielded lower apparent viscosity and lower yield stress [25]. Changes in such properties may influence how the melted chocolate spreads away from the capacitance plates, and thus affects the measured transition temperature. However, this would be expected to give lower measured transition temperatures. Thus, the influence of particle size on heat transfer and the resulting rate of melting are likely to be the predominant factors.
Table 7 shows how the peak capacitance temperature varied with fat content in the chocolate. Samples with greater fat content resulted in lower peak capacitance temperature (range 34.7 ± 0.3 to 39.7 ± 0.5 °C). This may be attributed to the fact that chocolates with greater fat content have lower apparent viscosity and yield stress, again making them easier to flow [26, 27]. These observations concurred with results from the dynamic rheology, which also indicated that higher fat content in chocolate samples produced lower Tonset.
Overall, samples with finer particles or lower fat content had lower peak capacitance values than those with larger particles and more fat. One of the critical factors that influences capacitance is the dielectric constant ε′ of the medium. The ε′ of cocoa butter (ε′ ~ 2.5) is higher than cocoa mass (ε′ ~ 1.5), so that higher fat content in chocolate samples will lead to higher absolute capacitance values. The way in which particles influence the dielectric properties of inhomogeneous media is complex. Several models have attempted to predict the complex permittivity of such media [28]. These models typically incorporate the ε′ and ε” values for each phase, the volume fraction (α), and in some cases the shape or size factors of suspended media. In general, increasing the volume fraction would tend to emphasize the properties of the suspended phase. Experimental studies suggest that these models perform better at lower α, and fail at some critical value at which percolation behavior becomes dominant. This critical value is thought to be dependent on particle size and shape.
Comparison of CTA, DSC and rheological data
The fat content and particles size had only a limited influence on melting temperatures as measured by DSC. In contrast, CTA measurements were more sensitive to variations in fat content and PSD. Rheological assessments of melting behavior were also more dependent on fat content and PSD. These observations reflect that melting has a thermodynamic component as well as a time-dependent element dependent on rates of heat transfer. Thus, the rheometer and CTA both require samples of greater mass as compared to that for DSC. Thus, DSC might be expected to give transition temperatures with greater precision, but may not totally reflect melting behavior as experienced by real world users of a product.
The transition temperatures measured by CTA were generally greater than those measured by DSC or rheometric techniques. However, a correlation between CTA values and the other methods was achieved. Several regression models were tested but the best fit, without overfitting, between Tm measured rheological and CTA methods was achieved with a quadratic model:
where a, b and c are fitted constants; Tm,CTA the melting temperature determined by the CTA device; and Tm,rhe the melting temperature determined by rheological methods. Equation 3 was used to model all data, that is, where particle size was varied or where fat content was varied. In addition, the model was applied using both the rheological onset and endset transition temperatures.
Figure 5 shows the results of the regression analyses. Both onset and endset temperatures were correlated to the CTA measured values. In the case of the onset temperature, the relationship was negative (r2 = 0.952) while for the endset temperature it was positive (r2 = 0.950). This does show that, if desired, the CTA method could be correlated with another technique.
This does raise the issue as to which approach provides the truest measure of melting point. For a pure substance, the Tm is more easily determined as melting occurs over a very sharp range. Chemists have often placed crystalline materials in capillary tubes and denoted where a sample visually melts as the temperature is increased slowly. In theory, a pure substance melts at one temperature, however there is always the need to impose a higher external temperature to allow heat transfer. Mixed crystalline systems, such as cocoa butter, melt over a broader range of temperature. This occurs as various distributions of fatty acids create fats with different melting fractions. In addition, fats may exist in one or more polymorphic forms that have different melting temperatures. These materials are best analyzed by scanning the temperature. The change in signal with time/temperature depends on the device. Thus the way that heat flux changes (in DSC) with solid fat ratio (as different fractions melt) is not identical to the way that molecular relaxations respond during thermal rheology.
Sample size and heating rate are other factors that skew the results. Samples with more mass take longer to melt, thus spread the melting process over a wider temperature range. However, overly small samples may hamper sensitivity. In general, lower heating rates should give truer results, but may compromise sensitivity and cause unacceptably long measurement times. All of the methods discussed are only as good as the temperature measurement. Thus, DSC, rheological or dielectric thermal devices need to be calibrated with materials of known melting properties.
The above factors also influence the various transition temperatures that are recorded. For example, the peak temperature often reported for DSC depends on both the sample size and heating rate. The CTA device was more sensitive to measuring the onset melting temperature than the other techniques studied, thus we expect it to be most beneficial to determine where melting begins.
Conclusions
The CTA device introduced in this study measured melting temperatures in chocolate with good repeatability. The device also showed some sensitivity to the effects of cocoa particle size and fat content on melting behavior. The instrument was inexpensive as it was built from metal plates connected to a capacitance meter, and did not require precise control of temperature ramping as used with DSC or dynamic thermal rheometers. While the measured temperatures were not identical to those from DSC or rheometric techniques, they could be easily correlated with those values if required.
References
E.O. Afoakwa, A. Paterson, M. Fowler, Factors influencing rheological and textural qualities in chocolate—a review. Trends Food Sci. Technol. 18, 290–298 (2007)
E.O. Afoakwa, A. Paterson, M. Fowler, M.J. Vieira, Influence of tempering and fat crystallization behaviours on microstructural and melting properties in dark chocolate systems. Food Res. Int 42, 200–209 (2009)
A.G. Stapley, H. Tewkesbury, P.J. Fryer, The effects of shear and temperature history on the crystallization of chocolate. J. Am. Oil Chem. Soc. 76, 677–685 (1999)
D. Dhonsi, A.G.F. Stapley, The effect of shear rate, temperature, sugar and emulsifier on the tempering of cocoa butter. J. Food Eng. 77, 936–942 (2006)
H. Schenk, R. Peschar, Understanding the structure of chocolate. Radiat. Phys. Chem. 71, 829–835 (2004)
E.O. Afoakwa, A. Paterson, M. Fowler, Effects of particle size distribution and composition on rheological properties of dark chocolate. Eur. Food Res. Technol. 226, 1259–1268 (2008)
C. Servais, R. Jones, I. Roberts, The influence of particle size distribution on the processing of food. J. Food Eng. 51, 201–208 (2002)
T.A. Do, J.M. Hargreaves, B. Wolf, J. Hort, J.R. Mitchell, Impact of particle size distribution on rheological and textural properties of chocolate models with reduced fat content. J. Food Sci. 72, E541-E552 (2007)
Y. Roos, Thermal analysis, state transitions and food quality. J. Therm. Anal. Calorim. 7, 197–203 (2003)
V. Glicerina, F. Balestra, M. Dalla Rosa, S. Romani, Rheological, textural and calorimetric modifications of dark chocolate during process. J. Food Eng. 119, 173–179 (2013)
Y. Wang, T.D. Wig, J. Tang, L.M. Hallberg, Dielectric properties of foods relevant to RF and microwave pasteurization and sterilization. J. Food Eng. 57, 257–268 (2003)
T.J. Laaksonen, Y.H. Roos, Thermal, dynamic-mechanical, and dielectric analysis of phase and state transitions of frozen wheat doughs. J. Cereal Sci 32, 281–292 (2000)
D. R. Lide ed., Permitivitty (dielectric constant) of gases in CRC Handbook of Chemistry and Physics, 86th edn. (CRC Press, Boca Raton, 2005), ISBN 0-8493-0486-5
P.A. Kilmartin, D.S. Reid, I. Samson, Dielectric properties of frozen maltodextrin solutions with added NaCl across the glass transition. J. Sci. Food Agric. 84, 1277–1284 (2004)
J. Tan, W.L. Kerr, Determination of glass transitions in boiled candies by capacitance based thermal analysis (CTA) and genetic algorithm (GA). J. Food Eng. 193, 68–75 (2017)
H.G. Merkus, G.M.H. Meesters, Particulate products: tailoring products for optimal performance (Springer, New York, 2013), pp. 253–273
S. Bolenz, A. Manske, Impact of fat content during grinding on particle size distribution and flow properties of milk chocolate. Eur. Food Res. Technol. 236, 863–872 (2013)
E.O. Afoakwa, A. Paterson, M. Fowler, J. Vieira, Characterization of melting properties in dark chocolates from varying particle size distribution and composition using differential scanning calorimetry. Food Res. Int. 41, 751–757 (2008)
S.T. Beckett, The Science of Chocolate, vol. 22 (Royal Society of Chemistry, Cambridge, 2000). pp. 81–101
L. Svanberg, L. Ahrné, N. Lorén, E. Windhab, Impact of pre-crystallization process on structure and product properties in dark chocolate. J. Food Eng. 114, 90–98 (2013)
C. Loisel, G. Keller, G. Lecq, B. Launay, M. Ollivon, Tempering of chocolate in a scraped surface heat exchanger. J. Food Sci. 62, 773–780 (1997)
C. Loisel, G. Lecq, G. Keller, M. Ollivon, Dynamic crystallization of dark chocolate as affected by temperature and lipid additives. J. Food Sci. 63, 73–79 (1998)
P. Lonchampt, R.W. Hartel, Fat bloom in chocolate and compound coatings. J. Lip. Sci. Technol. 106, 241–274 (2004)
A. Torbica, O. Jovanovic, B. Pajin, The advantages of solid fat content determination in cocoa butter and cocoa butter equivalents by the Karlshamns method. J. Food Res. Technol. 222, 385–391 (2006)
G. Mongia, G.,G.R. Ziegler, The role of particle size distribution of suspended solids in defining the flow properties of milk chocolate. Int. J. Food Prop. 3, 137–147 (2000)
M. Yanes, L. Durán, E. Costell, Rheological and optical properties of commercial chocolate milk beverages. J. Food Eng. 51, 229–234 (2002)
A. Sokmen, G. Gunes, Influence of some bulk sweeteners on rheological properties of chocolate. LWT Food Sci. Technol. 39, 1053–1058 (2006)
E.M. Kiley, V.V. Yakovlev, K. Ishizaki, S. Vaucher, Applicability study of classical and contemporary models for effective complex permittivity of metal powders. J. Microw. Power Electromagn. Energy 46, 26–38 (2012)
Acknowledgements
We would like to thank Dr. M. Balu and the CocoaTown Company in Atlanta, Georgia for their help with funding and acquisition of supplies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tan, J., Kerr, W.L. Determination of chocolate melting properties by capacitance based thermal analysis (CTA). Food Measure 12, 641–649 (2018). https://doi.org/10.1007/s11694-017-9677-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-017-9677-0