Abstract
This study was performed to determine bioactive compounds such as flavonoid and phenolic contents by HPLC and gas chromatography–mass spectrophotometry (GC–MS) and evaluate their antioxidant and antibacterial activities in bulb, flower and shoot extracts of Galanthus transcaucasicus Fomin. The overall results indicated that shoot part contained higher amounts of phenolics and flavonoids compared to the bulb and flower with respective values of 4.45and 2.67 mg/g DW. Besides, HPLC analyses showed that the gallic acid and syringic acid were the major phenolic contents in bulb, flower and shoot of G. transcaucasicus extracts. Apart from that, naringin was the main flavonoid compounds in bulb, flower and shoot. Volatile metabolites were detected by GC–MS screening that indicated 2-furancarboxaldehyde, 2,3-butanediol, and acetic acid as the main compounds in the bulb, flower and shoot extracts. The results of antioxidant activity test demonstrated that shoot extracts of G. transcaucasicus exhibited higher antioxidant activities compare to bulb and flower. Furthermore, the shoot, flower and bulb extracts showed high, moderate and weak antibacterial activities against common human and food borne pathogenic bacteria, respectively. The overall results revealed that various phytochemicals and bioactive compound in different parts of G. transcaucasicus could be considered as a source of putative antioxidant and antibacterial properties that could be used as biopreservative in food and cosmetic industries.
Similar content being viewed by others
Introduction
Phenolic and flavonoid compounds are a large group of secondary metabolites commonly found in plants. The phenolic acids commonly derived from shikimic acid, mostly occur in the bound form and their analgesic, antipyretic, cholagogic, sedative and anti-biotic properties are well documented [1]. The flavonoids are polyphenolic compounds, structurally derived from the flavones and are supposed to provide health benefits through radical scavenging and chelating activity. The anti-inflammatory, anti-allergic, anti-thrombitic, hepatoprotective, anti-spasmodic and anticancer properties of flavonoids such as rutin, kaempferol, quercetin, apigenin etc. are well-known [1, 2].
The Amaryllidaceae family as a member of monocotyledonous includes about 85 genera and 1100 species which grow in the region with the warm and mild weather all over the world [3]. In the last decade, phytochemical studies showed that there are a number of new compounds from alkaloids and phenolic combinations with diverse structures on some species of this plant family. Among these alkaloids, gracilines and plicamines constitute two new subgroups for the Amaryllidaceae alkaloids [4]. Several investigators reported strong antioxidant activity and high amount of phenolic, flavonoid compounds from Amaryllidaceae family plants such as Boophane disticha, Narcissus cultivars and Pancratium species [5,6,7,8] Also, acetylcholinesterase (AChE) inhibitory activity has been reported in Boophane disticha [5]. Galanthus genus from Amaryllidaceae family is bulbous perennial plants with ovoid to spherical bulb, narrow to linear green leaves, erect flowering stalks and white flowers and distributed around Europe through Anatolia and the Caucasus. Its eastern limit is the Caucasus and Iran and it extends Lebanon and South of Italy (Sicily) and Greece. Korkut [9] reported six different phenolic acids including p-hydroxy benzoic, vanillic and ferulic acids in Galanthus elwesii.
Galanthus transcaucasicusas Fomin is the only natural snowdrop species in Iran (Talysh and Alborz) that grows below sea level, occurring in the Hyrcanian forest along coast of the Caspian Sea. Also, G. transcaucasicus occurs in Azerbaijan, with a few records from Armenia [10]. More studies about the G. transcaucasicus compounds were extraction and characterization of lectins proteins as heterogeneous class of (g1yco) proteins [11]. Also, five kinds of isoquinoline alkaloids known as galanthamine (8.04%), narwedine (6.90%), lycorine (19.48%), caranine (3.45%) and tazettine (5.75%) were isolated from the alkaloid extract of the G. transcaucasicus bulbs [12]. Recently, Babashpour-Asl et al. [13] reported the alkaloid compounds of tissue-cultured bulbs of G. transcaucasicus plants. Also, identification of homolycorine and nerinine alkaloids from the bulbs of this plant has reported [13] and they suggested G. transcaucasicus as a new source of bioactive alkaloids.
While there are many reports on pharmacological activities of G. transcaucasicus plants proteins and alkaloids, there is little data related to phenolic compounds in G. transcaucasicus. Accordingly, this research was performed to determine the accumulation of secondary metabolite, including phenols and flavonoids and other bioactive compounds using HPLC and GC–MS in the bulb, shoot and flower of IRANIAN medicinal herb G. transcaucasicus. Furthermore, antioxidant properties of these compounds were assessed by DPPH, FRAP and ABTS and compared with some natural (i.e. vitamins C and E) and synthetic (i.e. BHT) antioxidants.
Materials and methods
Chemicals and reagents
The reagents including 1.1-diphenyl-2-picrylhydrazyl (DPPH), acetonitrile HPLC grade, alpha-tocopherol, aluminium chloride, ascorbic acid, butylated hydroxy toluene (BHT), dimethyl sulfoxide (DMSO), Folin–Ciocalteu reagent, hydrochloric acid, methanol, sodium carbonate, sodium hydroxide, purchased from Fisher Scientific, USA. All phenolics and flavonoids standard were purchased from Sigma-Aldrich. All other chemicals used in this experiment were purchased from Merck.
Plant materials and extract preparation
Galanthus transcaucasicus Fomin specimens were collected in March 2014 from the Alangdareh, Gorgan in the east of Alborz Mountain in Iran (longitude 36°47′ N and latitude 54°27′E, 350 m above sea level). Voucher specimens were identified by the Herbarium of Faculty of science, Golestan University, Gorgan, Iran, (G. transcaucasicus-(6100-GOL)). The different aerial parts of G. transcaucasicus (flower, shoot and bulb) were air-dried at ambient temperature (25 °C) for about 72 h and powdered using laboratory mill. Dried and powdered samples were extracted using methanol as a solvent as described by Crozier et al. [14].
Total phenolic and flavonoid assay
The total phenolic compounds (TPC) of the extracts were determined as described earlier by Hendra et al. [15]. The extract was measured at absorbance 765 nm and the result expressed as milligrams of gallic acid equivalents (GAE) per gram of dry matter. The total flavonoid compounds (TFC) was determined according to Ismail et al. [16]. The extract was measured using absorbance at 510 nm and the result was expressed as milligrams of rutin equivalents per gram of dry matter.
Determination of phenolic and flavonoid compounds by HPLC
To determine the quantity and types of phenolic and flavonoid compounds, the samples were analyzed by a high performance liquid chromatography (Waters, Milford, MA, USA) equipped with an analytical column (Inertsil ODS-3,5 μm 4.6 × 150 mm, Gl Science Inc) as described by Karimi et al. [17]. Phenolic and isoflavonoid compounds were detected at 280 nm while flavonoid compounds were detected at 350 nm. The phenolic compound standards used in this study were gallic acid, syringic acid, ferulic acid, catechin, epicatechin, ellagic acid and cinnamic acid and flavonoid and isoflavonoid standards were included quercetin, rutin, myricetin, apigenin, kampferol, daidezin, gentisic acid and naringin.
Gas chromatography–mass spectrophotometry method (GC–MS)
The GC–MS analysis of methanolic extract from different parts of G. transcaucasicus extracts were quantitatively performed by GC–MS (Shimadzu QP2010 PLUS system) equipped with a capillary column (30 m × 0.25 mm i.d. × 0.25 µm film thickness) based on the method described by Hossain and Rahman [18] with some modification.
Antioxidant activity
DPPH scavenging activity
The potential of the methanolic extracts for free radical scavenging activities were determined as described earlier by Gulcin et al. [19]. All measurements were performed in triplicates. The reaction mixture with lower absorbance values represents higher free radical scavenging activity. The free radical scavenging activities of the extracts were expressed as a percentage of inhibition and were calculated according to the following Eq. [20].
The A0 was the absorbance value of the blank sample or control reaction and A1 was the absorbance value of the test sample. A curve of percent inhibition or percent scavenging effect against samples concentrations was plotted and the concentration of the sample required for 50% inhibition was determined. The value for each of the test sample was presented as inhibition curve at 50% or IC50.
ABTS radical cation-scavenging
The ABTS radical scavenging activity of methanolic extracts was evaluated by Karimi et al. [21]. ABTS was dissolved in water, to a 7 ml concentration. ABTS radical cation (ABTS·+) was produced by reacting ABTS stock solution with 2.45 mM K2S2O8 and allowing the mixture to stand at room temperature (dark place) overnight before utilization.
Ferric-reducing antioxidant power (FRAP) assay
The ferric reducing property of the extracts was determined by using assay described by Yen and Chen [22]. One ml (concentration of 100, 150, 200, 250, and 300 μg/ml) of methanolic extract from different parts of G. transcaucasicus was mixed with 2.5 ml of potassium phosphate buffer (0.2 M, pH 6.6) and 2.5 ml of potassium ferricyanide (1 g/100 ml). The mixture was incubated at 50◦C for 25 min. Then, to stop the reaction the trichloroacetic acid (10%) was added to the mixture. An equal volume of distilled water was added followed by 0.5 ml ferum chlorate (0.1 g/100 ml) (FeCl3). The procedure was carried out in triplicate and allowed to stand for 30 min before measuring the absorbance at 700 nm. The above procedures were repeated with ascorbic acid, and BHT as the reference antioxidants. The percentage of antioxidant activity in FRAP assay of the samples was calculated according to the below formula:
A0 is the absorbance of the control (potassium phosphate buffer + FRAP reagent), A1 is the Absorbance of sample.
Antibacterial activity assay
The G. transcaucasicus bulb, flower and shoot methanolic extracts were evaluated for their antibacterial activity against Bacillus subtilis B145, Bacillus cereus B43, Staphylococcus aureus S1431, Escherichia coli E256 and Pseudomonas aeruginosa PI96 using disc diffusion method as described by Boussaada et al. [23]. All the bacteria were purchased from the Iranian Research Organization for Science and Technology and maintained in the Department of Microbiology, Faculty of Science, Islamic Azad University of Mashhad. The positive control without extracts (solvent) and reference antibiotic (kanamycin) were used in this test. The inhibitions of extracts were corrected based on inhibition caused by positive control and compared to the inhibition of reference antibiotic.
Statistical analysis
The data were analysed using a complete randomized design following the model: Yi = µ + Ti + ei, where µ is the mean, Ti is the treatment effect and ei is the experimental error, respectively. The means were compared using Duncan’s multiple range test and considered significant when p < 0.05.
Result and discussion
Total phenolic and flavonoid contents
Results on the phenolics and flavonoids contents (Table 1) in the bulb, flower and shoot extracts of G. transcaucasicus plants showed significant difference (p < 0.05). The results revealed that the shoot part contained higher phenolics and flavonoids compared to the bulb and flower with respective values of 4.45 mg gallic acid equivalents/g DW and 2.67 mg rutin equivalents/g DW (Table 1). Similarly, Khodadadi et al. [24] reported the higher amount of phenolics and flavonoids in shoot compared to bulb of Allium paradoxum. The amounts of phenolic and flavonoid compounds in bulbs of different populations of A. paradoxum in Iran were ranged from about 36 to 112 mg/g DW and from 1.3 to 7.7 mg/g DW for phenolic and flavonoid compounds, respectively which are higher from G. transcaucasicus. Also, the total phenolic (about 202 mg tannic acid/g) and flavonoids (about 9 mg quercetin/g) contents of Crinum bulbispermum (Amaryllidaceae) roots were higher from G. transcaucasicus [6].
Determination of phenolic and flavonoid compounds by HPLC
The concentrations of detected various phenolics and flavonoids from different aerial part of G. transcaucasicus methanolic extracts by HPLC are shown in the Tables 2 and 3. The obtain results confirmed the presence of gallic acid (439.5 µg/g DW) as the major phenolics in bulb and syringic acid (926.2, 705.5 µg/g DW) in flower and shoots of G. transcaucasicus plants, respectively. There is no report on amount of individual phenolics in G. transcaucasicus, however, free and esterified phenolic acids of G. elwesii were reported by Korkut [9]. They determined six phenolic acids including Cinnamic, ferulic, vanillic, p-coumaric, p-hydroxy benzoic, and caffeic acids that p-hydroxy benzoic was the major component among them followed by vanillic and ferulic acids [9]. Also, identification (and not quantification) of phenolic acids in ethanol extracts from five species of Amaryllidaceae including Sternbergia colchiciflora, G. nivalis, G. elwesii, Leucojum aestivum and Pancratium maritimum have reported by Nikolova and Gevrenova [25]. The presence of five phenolic acids e.g. coumaroyl-quinic, caffeic and ferulic acids in leaves and bulbous of Pancratium maritimum (Amaryllidaceae) have been reported [7]. Prakash et al. [26] have reported gallic acid, ferulic acid, protocatechuic acid, quercetin and kaempferol in different varieties of Allium cepa from India. Identification of six phenolic compounds was stated in the bulb extracts of Allium orientale in which caffeic acid (0.176 ± 0.008 mg/g) had in highest amount [27]. Jin et al. [28] have reported phenolic composition of bulbs of six Lilium species (L. regale, L. concolor, L. pumilum, L. leucanthum, L. davidii var. unicolor and L. lancifolium) native to China. Overall, rutin and kaempferol were the major phenolic compounds in lily bulb extracts, ranging from 0.96 to 20.98 and 1.30–12.48 mg/100 g DW, respectively.
The results indicated naringin was the only flavonoid compounds observed in all parts of G. transcaucasicus that their amounts were 58.3, 72.6 and 112.9 µg/g DW in bulb, flower and shoot of plants, respectively. In addition, Genistein as an isoflavonoid was also detected with respective values of 131.5 and 202 µg/g DW in flower and shoot part. This is the first report on HPLC analysis of individual phenolic and flavonoid compounds presence in G. transcaucasicus plants. The presence of 14 flavonols in leaves and bulbous parts in Pancratium maritimum (Amaryllidaceae) have been reported [7]. The major flavonols in P. maritimum were quercetin and isorhamnetin glycosides. Perez-Gregorio et al. [29] have reported eight flavonols from the edible part of the onion varieties where the major flavonols were quercetin 3,4-diglucoside and quercetin 4-glucoside. They found significant differences in total flavonoid concentrations were observed among the red and white varieties. The HPLC chromatogram in Fig. 1 shows the different phenolic compounds in the bulb of G. transcaucasicus and Fig. 2 indicates the different flavonoid compounds in the shoot of G. transcaucasicus as an instance.
GC–MS analysis of volatile compounds of G. transcaucasicus
Gas chromatography mass spectroscopy analysis was conducted in different plant parts and the crude methanolic extracts resulted in characterization of more than 128 compounds in the bulb, flower and shoot of G. transcaucasicus. The main and predominant volatile compounds were shown in Table 4. The overall results revealed that the main volatile compounds were acetic acid (13.6%), 2,3-Butanediol (43.13%) and 2-Furancarboxaldehyde (68.77%) in the shoot, flower and bulb extracts of G. transcaucasicus, respectively. Yousefbeyk et al. [12] reported that G. transcaucasicus contains biologically active alkaloids especially galanthamine and the major alkaloid lycorine, so it can be utilized in medicine.
Antioxidant activity assessment (DPPH, ABTS and FRAP)
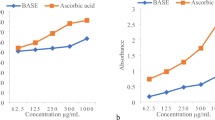
The results of antioxidant activities of extracts obtained from bulb, flower and shoot part of G. transcaucasicus in the reactions with DPPH, ABTS and FRAP assay, illustrated in Figs. 3, 4, 5, respectively. Our finding indicated the strong antioxidant activity in all parts of G. transcaucasicus plants. A steady increase was observed in the scavenging activity of free radicals in all the extracts in the range between 0 and 300 μg/ml. The IC50 concentrations were reported in Table 5. The assays of shoot extracts of G. transcaucasicus plants exhibited higher antioxidant activities DPPH IC50 = 125.07, ABTS IC50 = 238.27 and FRAP IC50 = 107.42 µg/ml compare to bulb and flower that concurred with the higher phenolic and flavonoid compounds in shoots (Table 1). It has been reported that generally in all plant species aerial parts had the strongest radical scavenging activity than bulbous parts [24]. The results indicated that antioxidant activities of the different aerials extracts were lower than reference antioxidant. Similarly, the antioxidant activity of ethanol extract of Narcissus cultivars and Boophane disticha plants (Amaryllidaceae) were (about 80 and 66% at the highest concentration tested) lower than those of the positive control [5, 8]. Adewusi and Steenkamp [6] reported antioxidant activates of 12 medicinal plants from southern Africa. The methanol or ethyl acetate extracts of Scadoxus puniceus (Amaryllidaceae) and Tulbaghia violacea (Alliaceae) plants showed either no activity or low radical scavenging activity (at highest tested concentration) in both the DPPH and ABTS assays. On the contrary, they stated good DPPH and ABTS radical scavenging ability in C. bulbispermum (Amaryllidaceae), Piper capense (Piperaceae), Lannea schweinfurthii plants (Anacardiaceae) [6]. The ability of radical scavenging of extracts could be related to the nature of phenolic and flavonoid compounds that allied to their electron transfer/hydrogen donating ability. Phenolic compounds are as radical scavengers, metal chelators, reducing agents, hydrogen donors and singlet oxygen quenchers. Also, the hydroxyl groups attached to the aromatic ring structures of flavonoids helps them scavenge free radicals [6]. Actually, the phenolic groups of flavonoids act as a source of ‘H’ atoms and the subsequent produced radicals can be de-localised over their structure [19].
Evaluation of antibacterial activity
The antibacterial potency of different aerial parts of G. transcaucasicus extracts were assessed by the presence or absence of inhibition zones (Table 6). The diameter of inhibition zones for bulb, flower and shoot extracts ranged from 0.35 to 0.85 cm, 0.76 to 1.22 cm and 0.92 to 1.29 cm at 0.5 mg/disc, respectively. Among bacteria, E. coli appeared to be the most sensitive and S. aureus was the most resistant strain bacteria against tested extracts.
Overall, the antibacterial activity of shoot extract appeared to be more effective than flower and bulb extract. The high antibacterial activity of shoot part may be due to the higher various bioactive compounds such as ferulic acid, kaempferol, genistein and 4H-Pyran-4-0ne compared to flower and bulb. Several phenolic acids such as gallic, caffeic and ferulic acids showed antibacterial activity against Gram-positive (S. aureus and L. monocytogenes) and Gram-negative bacteria (E. coli and P. aeruginosa) [30]. Also, the previous studies exhibited that the compounds containing pyran rings such as flavonoids possess antibacterial properties through induction of chemical barriers against invading microorganisms, disordering of nucleic acid synthesis, inactivation of adhesions and transport proteins [31]. Daglia [30] reported that kaempferol inhibit the catalytic activity of different bacterial topoisomerases. Thus, the antibacterial activity observed in the G. transcaucasicus extracts could only be attributed to the presence of phenolics, flavonoid and volatile compounds (Tables 2, 3, 4). The G. transcaucasicus extract is considered as a natural source of antibacterial compounds for the food industry. Naturally, derived preservatives can alter the taste of foods or exceed acceptable flavor thresholds [32].
Conclusion
Medicinal plants are known to have weak or strong therapeutic abilities and contribute in reducing risk of diseases and possess various biological activities. This is attributed to the large amounts of phytoconstituents such as flavonoids, phenolics and bioactive volatile compounds found in herbs. These bioactive compounds have received considerable attention due to their therapeutic potential for antimicrobial, anti-inflammatory, anticancer and antioxidant activities. This preliminary screening indicated the gallic acid and syringic acid were the main phenolics and naringin was the dominent flavonoid compounds in bulb flower and shoot of G. transcaucasicus methanol extracts. 2-furancarboxaldehyde, 2,3-butanediol and acetic acid were main volatile metabolites in this plant. Furthermore, the obtained results indicated G. transcaucasicus extracts specially its shoot has certain potential of antioxidant activity and as a source of antibacterial could be used as biopreservative in food and cosmetic industries.
References
T. Seal, Quantitative HPLC analysis of phenolic acids, flavonoids and ascorbic acid in four different solvent extracts of two wild edible leaves. Sonchus arvensis and Oenanthe linearis of North-Eastern region in India. J. App. Pharm. Sci 6, 157–166 (2016)
N.V. Thomas, S.K. Kim, Potential pharmacological applications of polyphenolic derivatives from marine brown algae. Environ. Toxicol. Pharmacol. 32, 325–335 (2011)
N. Lakshmi, Cytotaxonomical studies in eight genera of Amaryllidaceae. Cytologia 45, 663–673 (1980)
N. Unver, New skeletons and new concepts in Amaryllidaceae alkaloids. Phytochem. Rev. 6, 125–135 (2007)
E.A. Adewusi, G. Fouche, V. Steenkamp, Cytotoxicity and acetylcholinesterase inhibitory activity of an isolated crinine alkaloid from Boophane disticha (Amaryllidaceae). J. Ethnopharmacol 143, 572–578 (2012)
E.A. Adewusi, V. Steenkamp, In vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from southern Africa. Asian Pac. J. Trop. Med. 4, 829–835 (2011)
N. Rokbeni, Y. M’rabet, S. Cluzet, T. Richard, S. Krisa, M. Boussaid, A. Boulila, Determination of phenolic composition and antioxidant activities of Pancratium maritimum L. from Tunisia. Ind. Crops Prod. 94, 505–513 (2016)
X. Li, M. Lu, D. Tang, Y. Shi, Composition of carotenoids and flavonoids in Narcissus cultivars and their relationship with flower color. PLoS ONE. 10, e0142074 (2015)
A. Korkut, Research on variabilities in some important characters of Galanthus elwesii hook. Var. Elwesii grow under natural conditions. Acta Hortic 355, 189–194 (1994)
A.P. Davis, J.R. Barnett, The leaf anatomy of the genus Galanthus L. (Amaryllidaceae J. St.-Hil.). Bot. J. Linn. Soc 123, 333–352 (1997)
E.J. Damme, H. Kaku, F. Perini, I.J. Goldstein, B. Peeters, F. Yagi, B. Decock, W.J. Peumans, Biosynthesis, primary structure and molecular cloning of snowdrop (Galanthus nivalis L.) lectin. Eur. J. Biochem. 202, 23–30 (1991)
F. Yousefbeyk, B. Azadi, G. Amin, M. Salehi Sormaghi, M. Amini, M. Sharifzadeh, Phytochemical investigation of Galanthus transcaucasicus Fomin, as a source of isoquinoline alkaloids. Planta Med. 77, PG33 (2011)
M. Babashpour-Asl, H. Zakizadeh, H. Nazemiyeh, A. Motallebi-Azar, In vitro micropropagation and alkaloid production of Galanthus transcaucasicus Fomin. Pharm. Sci. 22, 267–271 (2016)
A. Crozier, M.E. Lean, M.S. McDonald, C. Black, Quantitative analysis of the flavonoid content of commercial tomatoes, onions, lettuce, and celery. J. Agric. Food Chem. 45, 590–595 (1997)
R. Hendra, S. Ahmad, E. Oskoueian, A. Sukari, M.Y. Shukor, Antioxidant, anti-inflammatory and cytotoxicity of Phaleria macrocarpa (Boerl.) Scheff fruit. BMC Complement. Altern. Med. 11, 1 (2011)
H.I. Ismail, K.W. Chan, A.A. Mariod, M. Ismail, Phenolic content and antioxidant activity of cantaloupe (Cucumis melo) methanolic extracts. Food Chem. 119, 643–647 (2010)
E. Karimi, H.Z. Jaafar, A. Ghasemzadeh, M. Ebrahimi, Fatty acid composition, antioxidant and antibacterial properties of the microwave aqueous extract of three varieties of Labisia pumila Benth. Biol. Res. 48, 9 (2015)
M.A. Hossain, A. Rahman, Chemical composition of bioactive compounds by GC–MS screening and anti-fungal properties of the crude extracts of cabbage samples. Asian J. Biotechnol. 3, 68–76 (2011)
I. Gulcin, I.G. Sat, S. Beydemir, M. Elmastas, O.I. Kufrevioglu, Comparison of antioxidant activity of clove (Eugenia caryophylata Thunb.) buds and lavender (Lavandula stoechas L.). Food Chem. 3, 393–400 (2004)
G.C. Yen, P.D. Duh, Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J. Agric. Food Chem. 42, 629–632 (1994)
E. Karimi, E. Oskoueian, R. Hendra, H.Z. Jaafar, Evaluation of Crocus sativus L. stigma phenolic and flavonoid compounds and its antioxidant activity. Molecules 5, 6244–6256 (2010)
G.C. Yen, H.Y. Chen, Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 43, 27–32 (1995)
O. Boussaada, J. Chriaa, R. Nabli, S. Ammar, D. Saidana, M.A. Mahjoub, I. Chraeif, A.N. Helal, Z. Mighri, Antimicrobial and antioxidant activities of methanol extracts of Evax pygmaea (Asteraceae) growing wild in Tunisia. World J. Microbiol. Biotechnol. 24, 1289–1296 (2008)
S. Khodadadi, T. Nejadsattari, A. Naqinezhad, M.A. Ebrahimzadeh, Diversity in antioxidant properties and mineral contents of Allium paradoxum in the Hyrcanian forests, Northern Iran. Biodiversitas 16, 281–287 (2015)
M. Nikolova, R. Gevrenova, Determination of phenolic acids in amaryllidaceae species by high performance liquid chromatography. Pharm. Biol. 43, 289–291 (2005)
D. Prakash, B.N. Singh, G. Upadhyay, Antioxidant and free radical scavenging activities of phenols from onion (Allium cepa). Food Chem. 102, 1389–1393 (2007)
O. Ceylan, H. Alıc, Antibiofilm, antioxidant, antimutagenic activities and phenolic compounds of Allium orientale BOISS. Braz. Arch. Biol. Technnol. 58, 935–943 (2015)
L. Jin, Y. Zhang, L. Yan, Y. Guo, L. Niu, Phenolic compounds and antioxidant activity of bulb extracts of six Lilium species native to China. Molecules 17, 9361–9378 (2012)
R.M. Perez-Gregorio, M.S. García-Falcón, J. Simal-Gándara, A.S. Rodrigues, D.P. Almeida, Identification and quantification of flavonoids in traditional cultivars of red and white onions at harvest. J. Food Comp. Anal. 23, 592–598 (2010)
M. Daglia, Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 23, 174–181 (2012)
T.P.T. Cushnie, A.J. Lamb, Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 26, 343–356 (2005)
A.I. Nazer, A. Kobilinsky, J.L. Tholozana, F. Dubois-Brissonneta, Combinations of food antimicrobials at low levels to inhibit the growth of Salmonella sv. Typhimurium: a synergistic effect? Food Micobiol. 22, 391–398 (2005)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Karimi, E., Mehrabanjoubani, P., Homayouni-Tabrizi, M. et al. Phytochemical evaluation, antioxidant properties and antibacterial activity of Iranian medicinal herb Galanthus transcaucasicus Fomin. Food Measure 12, 433–440 (2018). https://doi.org/10.1007/s11694-017-9656-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-017-9656-5