Abstract
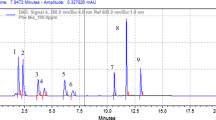
Phenolic compounds were extracted from seeds of eight varieties i.e. Rising Sun, SMH-0907, Ausigold-7, SMH-0939, US-444, Hysun-33, SMH-0917 and HS-K6 of sunflower and characterized by HPLC-DAD method. Quantification of individual compounds was carried out by external calibration. Total of 15 phenolic compound were identified, namely hydroxybenzoic acid, hydroxybenzoyl glucose, caftaric acid, rosmanol, gallic acid derivative, 3,4-di-O-caffeoylquinic acid, 3,5-di-O-caffeoylquinic acid, caffeic acid, pro-anthocynidin B1, rosmannic acid, sinapic acid derivative, caffeic acid derivative 1, caffeic acid derivative 2, caffeoylmalic acid derivative and quercetin derivative in the seeds of sunflower. Different treatments of potassium nitrate, zinc sulphate, and gibberellic acid showed significant effect on the biosynthesis of phenolic compound as compared to control plants. Among the 15 compounds analyzed 3,4-di-O-caffeoylquinic acid was predominant compound in the seeds. The current results demonstrate that this method can be efficiently used for the identification and quantification of phenolic compound in sunflower seeds.


Similar content being viewed by others
References
W. Wink, Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochem 64, 3–19 (2003)
A. Zeb, A reversed phase HPLC-DAD method for the determination of phenolic compounds in plant leaves. Anal. Methods 7, 7753–7757 (2015)
V. Lattanzio, P.A. Kroon, S. Quideau, D. Treutter, Plant phenolics secondary metabolites with diverse functions, vol. 1 (Blackwell Publishing, Hoboken, 2008) pp. 1–35 ()
A.J. Stangelo, Lipid oxidation in foods. Crit. Rev. Food Sci. Nutr. 36, 175–224 (1996)
N.J. Temple, Antioxidants and disease: more questions than answers. Nutr. Res. 20, 449–459 (2000)
B.L. Halvorsen, K. Holte, M.C.W. Myhrstad, I. Barikmo, E. Hvattum, S.F. Remberg, A.B. Wold, K. Haffner, H. Baugerod, L.F. Andersen, J.O. Moskaug, D.R. Jacobs, R. Blomhoff, A systematic screening of total antioxidants in dietary plants. J. Nutr. 132, 461–471 (2002)
S. Schmidt, J. Pokorny, Potential application of oilseeds as source of antioxidants for food. Czech J. Food Sci. 23, 93–102 (2005)
P.J. Caceres, C. Martınez-Villaluenga, L. Amigo, J. Frias, Food Chem. 152, 407–414 (2014)
A. Khoddami, M. Wilkes, T. Roberts, Techniques for analysis of plant phenolic compounds. Molecule 18, 2328–2375 (2013)
F.A. Tomas-Barberan, M.I. Gil, P. Cremin, A.L. Waterhouse, B. Hess-Pierce, A.A. Kader, J. Agric. Food Chem. 49, 4748–4760 (2001)
F.M. Pirisi, P. Cabras, C.F. Cao, M. Migliorini, M. Muggelli, J. Agric. Food. 48, 1191–1196 (2000)
J. Santos, M.B. Oliveira, E. Ibanez, M. Herrero, J. Chromatogr. 1327, 118–131 (2014)
P.F. Leal, N.B. Maia, Q.A.C. Caemello, P.R. Catharino, M.N. Eberlin, M.A. Meireles, Sweet basil extracts obtained by supercritical fluid extraction (SFE): global yields, chemical composition, antioxidant activity and estimation of the cost of manufacturing. Food Biopros. Technol. 1, 326–338 (2008)
G.D. Lecce, S. Arranz, O. Jauregui, A. Tresserra-Rimbau, P. Quifer-Rada, R.M. Lamuela-Ravent, Food Chem. 145, 874–882 (2014)
S.E. George, K. Ramalakshmi, L.J. Mohan-Rao, Crit. Rev. Food Sci. Nutr. 48, 464–469 (2008)
C. Jayasinghe, N. Gotoh, T. Aoki, S. Wada, Phenolics comparison and antioxidant activity of sweet basil. J. Agric. Food Chem. 51, 4442–4449 (2003)
P.M. Nguyen, E.M. Kwee, E.D. Niemeyer, Potassium rate alters the antioxidant capacity and phenolic concentration of basil leaves. Food Chem. 123(4), 1235–1241 (2010)
I. Fecka, S. Turek, Determination of polyphenolic compounds in commercial herbal drugs and spices from Lamiaceae: thyme, wild thyme and sweet marjoram by chromatographic techniques. Food Chem. 108, 1039–1053 (2008)
R.J. Grayer, G.C. Kite, N.C. Veitch, M.R. Eckert, P.D. Marin, P. Senanayake, Leaf flavonoid glycosides as chemosystematic characters in Ocimum. Biochem. Syst. Ecol. 30, 327–342 (2002)
M. Norhaiza, M. Maziah, M. Hakiman, Antioxidative properties of leaf extracts of popular Malaysian herb, Labisia pumila. J. Med. Plant Res. 3, 217–223 (2009)
A. Ghasemzadeh, H.Z.E. Jaafar, Effect of CO2 enrichment on synthesis of some primary and secondary metabolites in ginger. Int. J. Mol. Sci. 12, 1101–1114 (2011)
A. Ghasemzadeh, H.Z.A. Jaafar, A. Rahmat, P.E.M. Wahab, M.R.A. Halim, Effect of different light intensities on total phenolic and flavonoids synthesis and anti-oxidant activities in young ginger varieties. Int. J. Mol. Sci 11, 3885–3897 (2010)
H.L. Wei, E. Tye, D.F. Bresnick, Birt, Inhibitory effect of epigenin, a plant flavonoid, on epidermal ornithine decarboxylase and skin tumor promotion in mice. Cancer Res. 50, 499–502 (1990)
M.H. Ibrahim, H.Z.A. Jaafar, E. Karimi, A. Ghasemzadeh, Primary, secondary metabolites, photosynthetic capacity and antioxidant activity of the Malaysian Herb Kacip Fatimah exposed to potassium fertilization under greenhouse conditions. Int. J. Mol. Sci. 13, 15321–15342 (2012)
U. Krämer, S. Clemens, Functions and Homeostasis of Zinc, Copper, and Nickel in Plants. in Molecular Biology of Metal Homeostasis and Detoxification ed. by M.J. Tamás, E. Martinoia, (Springer, Berlin, 2006) pp. 215–271
S. Saadati, N. Moallemi, S.M.H. Mortazavi, S.M. Seyyed, Effects of zinc and boron foliar application on soluble carbohydrate and oil contents of three olive cultivars during fruit ripening. Sci. Hortic. 164, 30–34 (2013)
C. Song, M. Liu, J. Meng, M. Chi, Z. Xi, Z. Zhang, Promoting effect of foliage sprayed zinc sulfate on accumulation of sugar and phenolics in berries of Vitis vinifera cv. merlot growing on zinc deficient soil. Molecules 20, 2536–2554 (2015)
H. Zhao, C.C. Li, J. Pardo, P.C. Chu, C.X. Liao, J. Huang, J.G. Dong, X. Zhou, A novel E3 ubiquitin ligase TRAC-1 positively regulates T cell activation. J. Immun. 174(9), 5288–5297 (2005)
Z. Liang, M. Yini, X. Tao, C. Beimi, L. Yan, G. Zhixin, Y. Dongfeng, Effects of abscisic acid, gibberellin, ethylene and their interactions on production of phenolic acids in salvia miltiorrhiza bunge hairy roots. PLoS ONE 8(9), 72806–72812 (2013)
G.M. Weisz, D.R. Kammerer, R. Carle, Identification and quantification of phenolic compounds from sunflower (Helianthus annuus L.) kernels and shells by HPLC-DAD/ESI-MSn. Food Chem. 115, 758–765 (2009)
F.A. Tomás-Barberán, J.C. Espín, Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food and Agric. 81, 853–876 (2001)
Acknowledgements
We would like to acknowledge National Agriculture Research Centre (NARC) of Pakistan and grateful to Mr Amir Zia Scientific Officer in NARC who provided the seeds of sunflower varieties for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jan, A.U., Hadi, F., Zeb, A. et al. Identification and quantification of phenolic compounds through reversed phase HPLC-DAD method in sunflower seeds under various treatments of potassium nitrate, zinc sulphate and gibberellic acid. Food Measure 12, 269–277 (2018). https://doi.org/10.1007/s11694-017-9637-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-017-9637-8