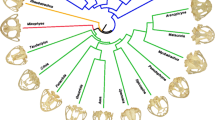
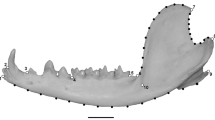
Abstract
Leaf-eating monkeys (colobines) are a highly diversified subfamily with 61 species in ten genera, in which patterns and constraints of morphological evolution are still poorly resolved. In the present study, we measured the skulls of 452 specimens collected from different museums worldwide. Using one of the most extensive samples ever employed, and geometric morphometric techniques, we aimed to elucidate the evolutionary processes that have led to the craniofacial diversification of colobines. Our comprehensive analyses of the colobine cranium demonstrated that phylogeny is the first order signal to emerge, with clear interspecific patterns of differentiation. Allometric trend constrains shape variation for most colobine taxa, but to a lesser degree than phylogeny. We also confirmed that diet is significantly associated with the variation in cranial shape among colobines. In particular, the mechanical advantage of the masseter for biting at the anterior dentition is linked to seed intake. We postulate that such ecomorphological patterns explain, in part, the non-phylogenetic and non-allometric variations in the colobine skull, and indicate the importance of diet in interspecific resource partitioning, allowing for species coexistence.







Similar content being viewed by others
References
Anderson, J., Cowlishaw, G., & Rowcliffe, J. M. (2007). Effects of forest fragmentation on the abundance of Colobus angolensis palliatus in Kenya’s Coastal Forests. International Journal of Primatology, 28, 637–655.
Auta, J., & Anwa, E. P. (2007). Preliminary studies on Albizzia lebbeck seeds: Proximate analysis and phytochemical screening. Research Journal of Biological Sciences, 2, 33–35.
Benefit, B. R., & McCrossin, M. L. (1991) Ancestral facial morphology of Old World higher primates. Proceedings of the National Academy of Sciences United States of America, 88, 5267–5271.
Bennett, E. L. (1983). The banded langur: Ecology of a colobine in West Malaysian rain-forest. Ph.D. thesis, University of Cambridge, Cambridge.
Bennett, E. L., & Davies, A. G. (1994). The ecology of Asian colobines. In A. G. Davies & J. F. Oates (Eds.), Colobine monkeys: Their ecology, behaviour and evolution (pp. 129–171). Cambridge: Cambridge University Press.
Bennett, E. L., & Sebastian, A. C. (1988). Social organization and ecology of proboscis monkeys (Nasalis larvatus) in mixed coastal forest in Sarawak. International Journal of Primatology, 9, 233–255.
Bergmann, C. (1848). Über die Verhältnisse der Wärme-ökonomie der Thiere zu ihrer Grösse. Göttinger Studien, 3, 595–708.
Bouvier, M. (1986). Biomechanical scaling of mandibular dimensions in New World monkeys. International Journal of Primatology, 7, 551–567.
Brugiere, D., Gautier, J. P., Moungazi, A., & Gautier-Hion, A. (2002). Primate diet and biomass in relation to vegetation composition and fruiting phenology in a rain forest in Gabon. International Journal of Primatology, 23, 999–1024.
Bruner, E. (2007). Cranial shape and size variation in human evolution: Structural and functional perspectives. Child’s nervous system, 23, 1357–1365.
Burness, G. P., Diamond, J., & Flannery, T. (2001). Dinosaurs, dragons, and dwarfs: The evolution of maximal body size. Proceedings of the National Academy of Sciences of the United States of America, 98, 14518–14523.
Cardini, A., & Elton, S. (2009). The radiation of red colobus monkeys (Primates, Colobinae): Morphological evolution in a clade of endangered African primates. Zoological Journal of the Linnean Society, 157, 197–224.
Chapman, C. A., Chapman, L. J., Cords, M., Gathua, J. M., Gautier-Hion, A., Lambert, J. E., Rode, K., Tutin, C. E. G., & White, L. J. T. (2004). Variation in the diets of Cercopithecus species: Differences within forests, among forests, and across species. In M. E. Glenn & M. Cords (Eds.), The guenons: Diversity and adaptation in African monkeys (pp. 325–350). New York: Kluwer Academic.
Chapman, C. A., Chapman, L. J., & Gillespie, T. R. (2002). Scale issues in the study of primate foraging: Red colobus of Kibale National Park. American Journal of Physical Anthropology, 117, 349–363.
Chatterjee, H. J., Ho, S. Y. W., Barnes, I., & Groves, C. (2009). Estimating the phylogeny and divergence times of primates using a supermatrix approach. BMC Evolutionary Biology, 9, 259.
Chivers, D. J., & Hladik, C. M. (1980). Morphology of the gastrointestinal tract in primates: Comparisons with other mammals in relation to diet. Journal of Morphology, 166, 337–386.
Clutton-Brock, T. H. (1975). Feeding behaviour of red colobus and black and white colobus in East Africa. Folia Primatologica, 23, 165–207.
Culhane, A. C., Perrière, G., Considine, E. C., Cotter, T. G., & Higgins, D. G. (2002). Between-group analysis of microarray data. Bioinformatics, 18, 1600–1608.
Curtin, S. H. (1976). Niche separation in sympatric Malaysian leaf-monkeys (Presbytis obscura and Presbytis melalophos). Yearbook of Physical Anthropology, 20, 421–439.
Curtin, S. H. (1980). Dusky and banded leaf monkeys. In D. J. Chivers (Ed.), Malayan forest primates: Ten years' study in tropical rain forest (pp. 105–145). New York: Plenum Press.
Daegling, D. J., Granatosky, M. C., McGraw, W. S., & Rapoff, A. J. (2011). Reduced stiffness of alveolar bone in the colobine mandible. American Journal of Physical Anthropology, 144, 421–431.
Daegling, D. J., & McGraw, W. S. (2001). Feeding, diet, and jaw form in West African Colobus and Procolobus. International Journal of Primatology, 22, 1033–1055.
Damuth, J., & MacFadden, B. J. (1990). Body size in mammalian paleobiology: Estimation and biological implications. Cambridge: Cambridge University Press.
Davies, A. G. (1991). Seed-eating by red leaf monkeys (Presbytis rubicunda) in dipterocarp forest of northern Borneo. International Journal of Primatology, 12, 119–144.
Davies, A. G., Bennett, E. L., & Waterman, P. G. (1988). Food selection by two South-east Asian colobine monkeys (Presbytis rubicunda and Presbytis melalophos) in relation to plant chemistry. Biological Journal of the Linnean Society, 34, 33–56.
Davies, A. G., Oates, J. F., & Dasilva, G. L. (1999). Patterns of frugivory in three West African colobine monkeys. International Journal of Primatology, 20, 327–357.
Dayan, T., & Simberloff, D. (1998). Size patterns among competitors: Ecological character displacement and character release in mammals,with special reference to island populations. Mammal Review, 28, 99–124.
Dela, J. D. S. (2007). Seasonal food use strategies of Semnopithecus vetulus nestor, at Panadura and Piliyandala, Sri Lanka. International Journal of Primatology, 28(3), 607–626.
Delson, E., Terranova, C. J., Jungers, W. L., Sargis, E. J., Jablonski, N. G., & Dechow, P. C. (2000). Body mass in Cercopithecidae (Primates, Mammalia): Estimation and scaling in extinct and extant taxa. Anthropological Papers of the American Museum of Natural History, 83, 1–159.
Drake, A., & Klingenberg, C. (2008) The pace of morphological change: Historical transformation of skull shape in St Bernard dogs. Proceedings of the Royal Society B: Biological Sciences, 275, 71–76.
Dray, S., & Dufour, A.-B. (2007). The ade4 package: Implementing the duality diagram for ecologists. Journal of Statistical Software, 22, 1–20.
Duc, H. M., Baxter, G. S., & Page, M. J. (2009). Diet of Pygathrix nigripes in southern Vietnam. International Journal of Primatology, 30, 15–28.
Ehlers Smith, D. A., Husson, S. J., Ehlers Smith, Y. C., & Harrison, M. E. (2013). Feeding ecology of red langurs in sabangau tropical peat-swamp forest, Indonesian Borneo: Extreme granivory in a non-masting forest. American Journal of Primatology, 75, 848–859.
Emerson, S. B., & Bramble, D. M. (1993). Scaling, allometry and skull design. In J. Hanken & B. K. Hall (Eds.), The skull (Vol. 3, pp. 384–421). Chicago: University of Chicago Press.
Felsenstein, J. (1985). Phylogenies and the comparative method. The American Naturalist, 125, 1–15.
Fimbel, C., Vedder, A., Dierenfeld, E., & Mulindahabi, F. (2001). An ecological basis for large group size in Colobus angolensis in the Nyungwe Forest, Rwanda. African Journal of Ecology, 39, 83–92.
Fleagle, J. G., & McGraw, W. S. (1999). Skeletal and dental morphology supports diphyletic origin of baboons and mandrills. Proceedings of the National Academy of Sciences of the United States of America, 96, 1157–1161.
Furuuchi, K., Koyabu, D., Mori, K., & Endo, H. (2013). Physiological cross-sectional area of the masticatory muscles in the giraffe (Giraffa camelopardalis). Mammal Study, 38, 67–71.
Groves, C. P. (2005). Subfamily Colobinae. In D. E. Wilson & D. M. Reeder (Eds.), Mammal species of the world (3rd ed., pp. 167–177). Baltimore: Johns Hopkins University Press.
Gupta, A. K., & Kumar, A. (1994). Feeding ecology and conservation of the Phayre’s leaf monkey Presbytis phayrei in northeast India. Biolological Conservation, 69, 301–306.
Hanya, G., & Bernard, H. (2012). Fallback foods of red leaf monkeys (Presbytis rubicunda) in Danum Valley, Borneo. International Journal of Primatology, 33, 322–337.
Hanya, G., & Bernard, H. (2015). Different roles of seeds and young leaves in the diet of red leaf monkeys (Presbytis rubicunda): Comparisons of availability, nutritional properties, and associated feeding behavior. International Journal of Primatology, 36, 177–193.
Harris, T. R., & Chapman, C. A. (2007). Variation in diet and ranging of black and white colobus monkeys in Kibale National Park, Uganda. Primates, 48, 208–221.
Huang, Z. P., Huo, S., Yang, S. G., Cui, L. W., & Xiao, W. (2010). Leaf choice in black-and-white snub-nosed monkeys Rhinopithecus bieti is related to the physical and chemical properties of leaves. Current Zoology, 56, 643–649.
Hull, D. B. (1979). A craniometric study of the black and white Colobus Illiger 1811 (Primates: Cercopithecoidea). American Journal of Physical Anthropology, 51, 163–181.
Hylander, W. L. (1979a). Mandibular function in Galago crassicaudatus and Macaca fascicularis: An in vivo approach to stress analysis of the mandible. Journal of Morphology, 159, 253–296.
Hylander, W. L. (1979b). The functional significance of primate mandibular form. Journal of Morphology, 160, 223–239.
Ito, K., & Endo, H. (2016). Comparative study of physiological cross-sectional area of masticatory muscles among species of Carnivora. Mammal Study, 41, 181–190.
Jablonski, N. G. (1998). The evolution of doucs and snub-nosed monkeys and the question of the phyletic unity of the odd-nosed colobines. In N. G. Jablonski (Eds.), The Natural History of the Doucs and Snub-Nosed Monkeys (pp. 13–52). Singapore: World Scientific.
Kamilar, J. M., & Paciulli, L. M. (2008). Examining the extinction risk of specialized folivores: A comparative study of colobine monkeys. American Journal of Primatology, 70, 816–827.
Karanth, K. P., Singh, L., Collura, R. V., & Stewart, C. B. (2008). Molecular phylogeny and biogeography of langurs and leaf monkeys of South Asia (Primates: Colobinae). Molecular Phylogenetics and Evolution, 46, 683–694.
Kay, R. F. (1975). The functional adaptations of primate molar teeth. American Journal of Physical Anthropology, 43, 195–215.
Kibaja, M. (2014). Diet of the ashy red colobus (Piliocolobus tephrosceles) and crop-raiding in a forest-farm mosaic, Mbuzi, Rukwa Region, Tanzania. Primate Conservation, 28, 109–116.
Kinzey, W. G. (1992). Dietary and dental adaptations in the Pitheciinae. American Journal of Physical Anthropology, 88, 499–514.
Kirkpatrick, R. C., & Grueter, C. C. (2010). Snub-nosed monkeys: Multilevel societies across varied environments. Evolutionary Anthropology, 19, 98–113.
Kool, K. M. (1993). The diet and feeding behavior of the silver leaf monkey (Trachypithecus auratus sondaicus) in Indonesia. International Journal of Primatology, 14, 667–700.
Koyabu, D., Oshida, T., Nguyen, S. T., Dang, C. N., Nguyen, N. X., Nguyen, D. X., Motokawa, M., Kimura, J., Sasaki, M., & Endo, H. (2012). Comparison of jaw muscle morphology in two sympatic callosciurine squirrels (Callosciurus erythraeus and Dremomys rufigenis) in Vietnam. Mammal Study, 37, 237–242.
Koyabu, D. B., & Endo, H. (2009). Craniofacial variation and dietary adaptations of African colobines. Journal of Human Evolution, 56, 525–536.
Koyabu, D. B., & Endo, H. (2010). Craniodental mechanics and diet in asian colobines: Morphological evidence of mature seed predation and sclerocarpy. American Journal of Physical Anthropology, 148, 137–148.
Langsrud, Ø., & Mevik, B. (2012). ffmanova: Fifty-fifty MANOVA. R package version 0.2-2. Retrieved from https://CRAN.R-project.org/package=ffmanova.
Lavrenchenko, L. a. (2014). Hybrid speciation in mammals: Illusion or reality? Biology Bulletin Reviews, 4, 198–209.
Ledevin, R., Quéré, J.-P., Michaux, J. R., & Renaud, S. (2012). Can tooth differentiation help to understand species coexistence? The case of wood mice in China. Journal of Zoological Systematics and Evolutionary Research, 50, 315–327.
Li, B., Pan, R., & Oxnard, C. E. (2002). Extinction of snub-nosed monkeys in China during the past 400 years. International Journal of Primatology, 23, 1227–1244.
Li, Y., Jiang, Z., Li, C., & Grueter, C. C. (2010). Effects of seasonal folivory and frugivory on ranging patterns in Rhinopithecus roxellana. International Journal of Primatology, 31, 609–626.
Liedigk, R., Yang, M., Jablonski, N. G., Momberg, F., Geissmann, T., Lwin, N., Hla, T. H., Liu, Z., Wong, B., Ming, L., Yongcheng, L., Zhang, Y. P., Nadler, T., Zinner, D., & Roos, C. (2012) Evolutionary history of the odd-nosed monkeys and the phylogenetic position of the newly described myanmar snub-nosed monkey Rhinopithecus strykeri. PLoS ONE, 7, e37418.
Liu, X., Stanford, C. B., Yang, J., Yao, H., & Li, Y. (2013). Foods eaten by the sichuan snub-nosed monkey (Rhinopithecus roxellana) in Shennongjia National Nature Reserve, China, in relation to nutritional chemistry. American Journal of Primatology, 75, 860–871.
Lomolino, M. V. (1985). Body size of mammals on islands: The island rule reexamined. The American Naturalist, 125, 310–316.
Lucas, P. W. (2004) Dental functional morphology: How teeth work (pp. 1–355). Cambridge: Cambridge University Press.
Lucas, P. W., Beta, T., Darvell, B. W., Dominy, N. J., Essackjee, H. C., Lee, P. K. D., & Yuen, T. D. B. (2001). Field kit to characterize physical, chemical and spatial aspects of potential primate foods. Folia Primatologica, 72, 11–25.
Lucas, P. W., Copes, L., Constantino, P. J., Vogel, E. R., Chalk, J., Talebi, M., Landis, M., & Wagner, M. (2012). Measuring the toughness of primate foods and its ecological value. International Journal of Primatology, 33, 598–610.
Lucas, P. W., & Teaford, M. F. (1994). Functional morphology of colobine teeth. In A. G. Davies & J. F. Oates (Eds.), Colobine monkeys: Their ecology, behaviour and evolution (pp. 173–203). Cambridge: Cambridge University Press.
Lucas, P. W., Turner, I. M., Dominy, N. J., & Yamashita, N. (2000). Mechanical defences to herbivory. Annals of Botany, 86, 913–920.
Maddison, W. P., & Maddison, D. R. (2011) Mesquite: A modular system for evolutionary analysis. Version 2.75. Retrieved from http://mesquiteproject.org
Maisels, F., Gautier-Hion, A., & Gautier, J.-P. (1994). Diets of two sympatric colobines in Zaire: More evidence on seed-eating in forests on poor soils. International Journal of Primatology, 15, 681–701.
Marroig, G., Shirai, L. T., Porto, A., de Oliveira, F. B., & De Conto, V. (2009). The evolution of modularity in the mammalian skull II: Evolutionary consequences. Evolutionary Biology, 36, 136–148.
Marsh, C. W. (1981). Diet choice among Red Colobus (Colobus badius rufomitratus) on the Tana River, Kenya. Folia Primatologica, 35(2–3), 147–178.
Matsuda, I., Tuuga, A., Bernard, H., Sugau, J., & Hanya, G. (2013). Leaf selection by two Bornean colobine monkeys in relation to plant chemistry and abundance. Scientific Reports, 3, 1873.
Matsuda, I., Tuuga, A., Hashimoto, C., Bernard, H., Yamagiwa, J., Fritz, J., Tsubokawa, K., Yayota, M., Murai, T., Iwata, Y., & Clauss, M. (2014). Faecal particle size in free-ranging primates supports a ‘rumination’strategy in the proboscis monkey (Nasalis larvatus). Oecologia, 174, 1127–1137.
Matsuda, I., Tuuga, A., & Higashi, S. (2009). The feeding ecology and activity budget of proboscis monkeys. American Journal of Primatology, 71, 478–492.
McGraw, W. S., van Casteren, A., Kane, E., Geissler, E., Burrows, B., & Daegling, D. J. (2016). Feeding and oral processing behaviors of two colobine monkeys in Tai Forest, Ivory Coast. Journal of Human Evolution, 98, 90–102.
McKey, D. B. (1978). Soils, vegetation, and seed-eating by black colobus monkeys. In G. Montgomery (Ed.), The ecology of arboreal folivores (pp. 423–437). Washington D.C: Smithsonian Institution Press.
McKey, D. B., Gartlan, J. S., Waterman, P. G., & Choo, G. M. (1981). Food selection by black colobus monkeys (Colobus satanas) in relation to plant chemistry. Biological Journal of the Linnean Society, 16, 115–146.
Md-Zain, B. M., Hasan, M. H., & Melnick, D. J. (2002) Defining evolutionary significant units for Presbytis melalophos conservation using mitochondrial DNA sequences. In Proceedings of the regional symposium on environment and natural resources, (Vol. 1, pp. 279–286).
Meijaard, E., & Groves, C. P. (2004) The biogeographical evolution and phylogeny of the genus Presbytis. Primate Report, 68, 71–90.
Meiri, S., & Dayan, T. (2003). On the validity of Bergmann’s rule. Journal of Biogeography, 30, 331–351.
Meloro, C., Cáceres, N., Carotenuto, F., Sponchiado, J., Melo, G. L., Passaro, F., & Raia, P. (2014). In and out the Amazonia: Evolutionary ecomorphology in howler and capuchin monkeys. Evolutionary Biology, 41, 38–51.
Meyer, D., Rinaldi, I. D., Ramlee, H., Perwitasari-Farajallah, D., Hodges, J. K., & Roos, C. (2011). Mitochondrial phylogeny of leaf monkeys (genus Presbytis, Eschscholtz, 1821) with implications for taxonomy and conservation. Molecular Phylogenetics and Evolution, 59, 311–319.
Milton, K. (1979). Factors influencing leaf choice by howler monkeys: A test of some hypotheses of food selection by generalist herbivores. The American Naturalist, 114, 362–378.
Minhós, T., Sousa, C., Vicente, L. M., & Bruford, M. W. (2015). Kinship and intragroup social dynamics in two sympatric African colobus species. International Journal of Primatology, 36, 871–886.
Mitteroecker, P., Gunz, P., Bernhard, M., Schaefer, K., & Bookstein, F. L. (2004). Comparison of cranial ontogenetic trajectories among great apes and humans. Journal of Human Evolution, 46, 679–698.
Mturi, F. A. (1993). Ecology of the Zanzibar red colobus monkey, Colobus badius kirkii (Gray, 1968), in comparison with other red colobines. In J. C. Lovett & S. K. Wasser (Eds.), Biogeography and ecology of the rain forest of eastern Africa (pp. 243–266). Cambridge: Cambridge University Press.
Nowak, K., Cardini, A., & Elton, S. (2008). Evolutionary acceleration and divergence in Procolobus kirkii. International Journal of Primatology, 29, 1313.
Oates, J. F. (1988). The diet of the olive colobus monkey, Procolobus verus, in Sierra Leone. International Journal of Primatology, 9, 457–478.
Oates, J. F. (1994). The natural history of African colobines. Colobine monkeys: Their ecology, behaviour and evolution. In A. G. Davies & J. F. Oates (Eds.), Colobine monkeys: Their ecology, behaviour and evolution (pp. 75–128). Cambridge: Cambridge University Press.
Osterholz, M., Walter, L., & Roos, C. (2008). Phylogenetic position of the langur genera Semnopithecus and Trachypithecus among Asian colobines, and genus affiliations of their species groups. BMC Evolutionary Biology, 8, 58.
Pan, R. (2006). Dental morphometric variation between African and Asian colobines, with special reference to the other Old World monkeys. Journal of Morphology, 267, 1087–1098.
Paradis, E., Claude, J., & Strimmer, K. (2004). APE: Analyses of phylogenetics and evolution in R language. Bioinformatics, 20, 289–290.
Parra, R. (1978). Comparison of foregut and hindgut fermentation in herbivores. In G. Montgomery (Ed.), The ecology of arboreal folivores (pp. 205–230). Washington D.C.: Smithsonian Institution Press.
Pohlert, T. (2016). The pairwise multiple comparison of mean ranks package (PMCMR). Resource document. Retrieved July 26, 2017, from https://cran.r-project.org/web/packages/PMCMR/vignettes/PMCMR.pdf.
Quyet, L. K., Duc, N. A., Tai, V. A., Wright, B. W., & Covert, H. H. (2007). Diet of the Tonkin snub-nosed monkey (Rhinopithecus avunculus) in the Khau Ca area, Ha Giang Province, northeastern Vietnam. Vietnamese Journal of Primatology, 1, 75–83.
Raadsheer, M. C., Van Eijden, T., Van Ginkel, F. C., & Prahl-Andersen, B. (1999). Contribution of jaw muscle size and craniofacial morphology to human bite force magnitude. Journal of Dental Research, 78, 31–42.
Raia, P., & Meiri, S. (2006). The island rule in large mammals: Paleontology meets ecology. Evolution, 60, 1731–1742.
Ravosa, M. J. (1990). Functional assessment of subfamily variation in maxillomandibular morphology among Old World monkeys. American Journal of Physical Anthropology, 82, 199–212.
Ravosa, M. J. (1996). Jaw morphology and function in living and fossil Old World monkeys. International Journal of Primatology, 17, 909–932.
Ravosa, M. J., Noble, V. E., Hylander, W. L., Johnson, K. R., & Kowalski, E. M. (2000). Masticatory stress, orbital orientation and the evolution of the primate postorbital bar. Journal of Human Evolution, 38, 667–693.
Rawson, B. (2009). The socio-ecology of the black-shanked douc (Pygathrix nigripes) in Mondulkiri Province. Ph.D. thesis, The Australian National University.
R-Core-Team (2016). R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. Resource document. Retrieved July 26, 2017, from http://www.R-project.org/.
Renaud, S., Dufour, A., Hardouin, E. A., & Ledevin, R. (2015). Once upon multivariate analyses: When they tell several stories about biological evolution. PLoS ONE, 10, 1–18.
Revell, L. J. (2012). phytools: An R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution, 3, 217–223. https://doi.org/10.1111/j.2041-210X.2011.00169.x.
Rilling, J. K., & a Seligman, R. (2002). A quantitative morphometric comparative analysis of the primate temporal lobe. Journal of Human Evolution, 42, 505–533.
Rode, K. D., Chapman, C. A., Chapman, L. J., & McDowell, L. R. (2003). Mineral resource availability and consumption by colobus in Kibale national park, Uganda. International Journal of Primatology, 24, 541–573.
Rohlf, F. J., & Corti, M. (2000). Use of two-block partial least-squares to study covariation in shape. Systematic Biology, 49, 740–753.
Rohlf, F. J., & Slice, D. (1990). Extensions of the procrustes method for the optimal superimposition of landmarks. Systematic Zoology, 39, 40–59.
Roos, C., Nadler, T., & Walter, L. (2008). Mitochondrial phylogeny, taxonomy and biogeography of the silvered langur species group (Trachypithecus cristatus). Molecular Phylogenetics and Evolution, 47, 629–636.
Ruhiyat, Y. (1983). Socio-ecological study of Presbytis aygula in West Java. Primates, 24, 344–359.
Schlager, S. (2013). Package ‘Morpho’. Resource document. Retrieved July 26, 2017, from https://cran.r-project.org/web/packages/Morpho/Morpho.pdf.
Scott, R. S., Teaford, M. F., & Ungar, P. S. (2012). Dental microwear texture and anthropoid diets. American Journal of Physical Anthropology, 147, 551–579.
Simberloff, D., Dayan, T., Jones, C., & Ogura, G. (2000). Character displacement and release in the small Indian mongoose, Herpestes javanicus. Ecology, 81, 2086–2099.
Sinclair, A. R. E., Mduma, S., & Brashares, J. S. (2003). Patterns of predation in a diverse predator–prey system. Nature, 425, 288–290.
Singleton, M. (2002). Patterns of cranial shape variation in the Papionini (Primates: Cercopithecinae). Journal of Human Evolution, 42, 547–578.
Singleton, M. (2005). Functional shape variation in the cercopithecine masticatory complex. In D. E. Slice (Ed.), Modern morphometrics in physical anthropology (pp. 319–348). New York: Kluwer Academic/Plenum Publishers.
Snaith, T. V., & Chapman, C. A. (2008). Red colobus monkeys display alternative behavioral responses to the costs of scramble competition. Behavioral Ecology, 19, 1289–1296.
Sokal, R. R., & Rohlf, F. J. (2012) Biometry (4th ed.). New York: WH Freeman and Company.
Sondaar, P. Y. (1977). Insularity and its effect on mammal evolution. In M. K. Hecht, P. C. Goody & B. M. Hecht (Eds.), Major patterns in vertebrate evolution (pp. 671–707). New York: Plenum.
Spencer, M. A., & Demes, B. (1993). Biomechanical analysis of masticatory system configuration in Neandertals and Inuits. American Journal of Physical Anthropology, 91, 1–20.
Stanford, C. B. (1991). The diet of the capped langur (Presbytis pileata) in a moist deciduous forest in Bangladesh. International Journal of Primatology, 12, 199–216.
Steudel, K. (1982). Patterns of intraspecific and interspecific allometry in Old World primates. American Journal of Physical Anthropology, 59, 419–430.
Starin, E. D. (1991). Socioecology of the red colobus monkey in the Gambia with partial reference to female-male differences and transfer patterns. Ph.D. thesis, City University of New York, New York.
Struhsaker, T. T. (1975). The red colobus monkey. Chicago: University of Chicago Press.
Sunderraj, S. F. W. (2001). Ecology and conservation of Nilgiri langur (Trachypithecus johnii). Envis Bulletin: Wildlife and Protected Areas, 1, 49–59.
Swindler, D. R. (1976). Dentition of living primates. London: Academic Press.
Taylor, A. B., & Vinyard, C. J. (2009). Jaw-muscle fiber architecture in tufted capuchins favors generating relatively large muscle forces without compromising jaw gape. Journal of Human Evolution, 57, 710–720.
Teaford, M. F. (1983). The morphology and wear of the lingual notch in macaques and langurs. American Journal of Physical Anthropology, 60, 7–14.
Teichroeb, J. A., Saj, T. L., Paterson, J. D., & Sicotte, P. (2003). Effect of group size on activity budgets of Colobus vellerosus in Ghana. International Journal of Primatology, 24, 743–758.
Ting, N. (2008). Mitochondrial relationships and divergence dates of the African colobines: Evidence of Miocene origins for the living colobus monkeys. Journal of Human Evolution, 55, 312–325.
Ting, N., Tosi, A. J., Li, Y., Zhang, Y.-P., & Disotell, T. R. (2008). Phylogenetic incongruence between nuclear and mitochondrial markers in the Asian colobines and the evolution of the langurs and leaf monkeys. Molecular phylogenetics and evolution, 46, 466–474.
Ungar, P. (1998). Dental allometry, morphology, and wear as evidence for diet in fossil primates. Evolutionary Anthropology, 6, 205–217.
Vandercone, R. P., Dinadh, C., Wijethunga, G., Ranawana, K., & Rasmussen, D. T. (2012). Dietary diversity and food selection in Hanuman langurs (Semnopithecus entellus) and purple-faced langurs (Trachypithecus vetulus) in the Kaludiyapokuna Forest Reserve in the dry zone of Sri Lanka. International Journal of Primatology, 33, 1382–1405.
Van Valen, L. (1973). Pattern and the balance of nature. Evolutionary Theory, 1, 31–49.
Verheyen, W. N. (1962) Contribution à la craniologie comparée des primates: les genres Colobus Illiger 1811 et Cercopithecus Linné 1758. Musée royal de l’Afrique centrale.
Wang, B., Zhou, X., Shi, F., Liu, Z., Roos, C., Garber, P. A., Li, M., & Pan, H. (2015). Full-length Numt analysis provides evidence for hybridization between the Asian colobine genera Trachypithecus and Semnopithecus. American Journal of Primatology, 77, 901–910.
Watanabe, K. (1981). Variations in group composition and population density of the two sympatric Mentawaian leaf-monkeys. Primates, 22, 145–160.
Waterman, P. G., & Kool, K. M. (1994). Colobine food selection and plant chemistry. In A. G. Davies & J. F. Oates (Eds.), Colobine monkeys: Their ecology, behaviour and evolution (pp. 251–284). Cambridge: Cambridge University Press.
Williams, S. H., Wright, B. W., Truong, V., Daubert, C. R., & Vinyard, C. J. (2005). Mechanical properties of foods used in experimental studies of primate masticatory function. American Journal of Primatology, 67, 329–346.
Wright, B. W. (2005). Craniodental biomechanics and dietary toughness in the genus Cebus. Journal of Human Evolution, 48, 473–492.
Wright, B. W., Ulibarri, L., O’brien, J., Sadler, B., Prodhan, R., Covert, H. H., & Nadler, T. (2008). It’s tough out there: Variation in the toughness of ingested leaves and feeding behavior among four Colobinae in Vietnam. International Journal of Primatology, 29, 1455–1466.
Wright, B. W., & Willis, M. S. (2012). Relationships between the diet and dentition of Asian leaf monkeys. American Journal of Physical Anthropology, 148, 262–275.
Yamashita, N., Cuozzo, F. P., Sauther, M. L., Fitzgerald, E., Riemenschneider, A., & Ungar, P. S. (2016). Mechanical food properties and dental topography differentiate three populations of Lemur catta in southwest Madagascar. Journal of Human Evolution, 98, 66–75.
Yan-Zhang, P., Ru-Liang, P., & Jablonski, N. G. (1993). Classification and evolution of Asian colobines. Folia Primatologica, 60, 106–117.
Zhou, Q., Wei, F., Li, M., Huang, C., & Luo, B. (2006). Diet and food choice of Trachypithecus francoisi in the Nonggang Nature Reserve, China. International Journal of Primatology, 27, 1441–1460.
Zollikofer, C. P. E., & Ponce de León, M. S. (2010). The evolution of hominin ontogenies. Seminars in Cell & Developmental Biology, 21, 441–452.
Acknowledgements
Authors thank the museum staffs for access to specimens and Goro Hanya, Takeshi Nishimura, Gen Suwa, Daisuke Shimizu, and Masanaru Takai for discussions. This study was supported by KAKENHI (Grant Nos. 26711023, 18H02492, 18H04816, and 18K19359), by the Cooperation Research Program of Primate Research Institute, Kyoto University (Grant No. 2010-A-3) and by the LabEx Sciences Archéologiques de Bordeaux (Grant No. ANR-10-LABX-52).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11692_2019_9469_MOESM1_ESM.xlsx
Online Resource 1 List of the specimens analyzed in this study, with accession ID and institutes housing them: Natural History Museum (BMNH), Primate Research Institute of Kyoto University (KUPRI), Smithsonian National Museum of Natural History (USNM), and Zoological Reference Collection of Lee Kong Chian Natural History Museum at the National University of Singapore (ZRC)—Supplementary material 1 (XLSX 32 KB)
11692_2019_9469_MOESM3_ESM.xlsx
Online Resource 3 Landmark definitions employed in the geometric morphometric analyses—Supplementary material 3 (XLSX 9 KB)
11692_2019_9469_MOESM4_ESM.xlsx
Online Resource 4 Mean values of measured moment arms and calculated mechanical advantages of the masseter, temporalis, and medial pterygoid muscles for each species. Canine position measurement is not available for T. policephalus due to missing canine in the studied specimen—Supplementary material 4 (XLSX 23 KB)
11692_2019_9469_MOESM5_ESM.xlsx
Online Resource 5 Pearson’s correlation coefficients for comparsions between mechanical advantage and centroid size—Supplementary material 5 (XLSX 9 KB)
11692_2019_9469_MOESM6_ESM.xls
Online Resource 6 Inter-specific comparison of size using Kruskal-Wallis tests. Significant probabilities are either in bold (P < 0.001) or in italic (P < 0.05). Species for which the number of specimens available if lower than five are written in red—Supplementary material 6 (XLS 51 KB)
11692_2019_9469_MOESM7_ESM.xlsx
Online Resource 7 Basic measurements (n = 15) and centroid size (n = 2) for Simias concolor—Supplementary material 7 (XLSX 9 KB)
11692_2019_9469_MOESM8_ESM.pdf
Online Resource 8 Alternative representation of shape deformations associated to: 1) shape axes bgPC1, bgPC2 and bgPC3, 2) allometry, and 3) 2B-PLS. Here the red dots correspond to the reference configuration compared to the target configuration (in green). The line between each red dot and its homologous green dot represents the deformation—Supplementary material 8 (PDF 1894 KB)
Rights and permissions
About this article
Cite this article
Ledevin, R., Koyabu, D. Patterns and Constraints of Craniofacial Variation in Colobine Monkeys: Disentangling the Effects of Phylogeny, Allometry and Diet. Evol Biol 46, 14–34 (2019). https://doi.org/10.1007/s11692-019-09469-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-019-09469-7