Abstract
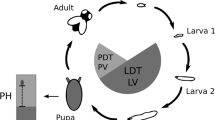
Characterizing the relationships between genotype and phenotype for developmental adaptive traits is essential to understand the evolutionary dynamics underlying biodiversity. In holometabolous insects, the time to reach the reproductive stage and pupation site preference are two such traits. Here we characterize aspects of the genetic architecture for Developmental Time (decomposed in Larval and Pupal components) and Pupation Height using lines derived from three natural populations of Drosophila melanogaster raised at two temperatures. For all traits, phenotypic differences and variation in plasticity between populations suggest adaptation to the original thermal regimes. However, high variability within populations shows that selection does not exhaust genetic variance for these traits. This could be partly explained by local adaptation, environmental heterogeneity and modifications in the genetic architecture of traits according to environment and ontogenetic stage. Indeed, our results show that the genetic factors affecting Developmental Time and Pupation Height are temperature-specific. Varying relationships between Larval and Pupal Developmental Time between and within populations also suggest stage-specific modifications of genetic architecture for this trait. This flexibility would allow for a somewhat independent evolution of adaptive traits at different environments and life stages, favoring the maintenance of genetic variability and thus sustaining the traits’ evolvabilities.





Similar content being viewed by others
References
Angilletta, M., Steury, T., & Sears, M. (2004). Temperature, growth rate, and body size in ectotherms: Fitting pieces of a life-history puzzle. Integrative and Comparative Biology, 44, 498–509.
Arbeitman, M. N., Furlong, E. E., Imam, F., Johnson, E., Null, B. H., et al. (2002). Gene expression during the life cycle of Drosophila melanogaster. Science, 297, 2270–2275.
Artieri, C. G., & Singh, R. S. (2010). Molecular evidence for increased regulatory conservation during metamorphosis, and against deleterious cascading effects of hybrid breakdown in Drosophila. BMC Biology, 8, 26.
Atchley, W. R., Gaskins, C. T., & Anderson, D. (1976). Statistical properties of ratios. I. Empirical results. Systematic Biology, 25, 137–148.
Bauer, S. J., & Sokolowski, M. B. (1985). A genetic analysis of path length and pupation height in a natural population of Drosophila melanogaster. Canadian Journal of Genetics and Cytology, 27, 334–340.
Beltramí, M., Medina-Muñoz, M. C., Arce, D., & Godoy-Herrera, R. (2010). Drosophila pupation behavior in the wild. Evolutionary Ecology, 24, 347–358.
Bharathi, N. S., Prasad, N. G., Shakarad, M., & Joshi, A. (2004). Correlates of sexual dimorphism for dry weight and development time in five species of Drosophila. Journal of Zoology, 264, 87–95.
Bryant, E. H. (1969). A system favoring evolution of holometabolous development. Annals of the Entomological Society of America, 62, 1087–1091.
Bürger, R., & Gimelfarb, A. (2002). Fluctuating environments and the role of mutation in maintaining quantitative genetic variation. Genetics Research, 80, 31–46.
Carreira, V. P., Imberti, M., Mensch, J., & Fanara, J. J. (2013). Gene-by-temperature interactions and candidate plasticity genes for morphological traits in Drosophila melanogaster. PLoS ONE, 8(7), e70851.
Casares, P., & Carracedo, M. C. (1987). Pupation height in Drosophila: Sex differences and influence of larval developmental time. Behavior Genetics, 17, 523–535.
Chippindale, A. K., Alipaz, J. A., Chen, H. W., & Rose, M. R. (1997). Experimental evolution of accelerated development in Drosophila. 1. Developmental speed and larval survival. Evolution, 51(5), 1536–1551.
Chippindale, A. K., Alipaz, J. A., & Rose, M. R. (2004). Experimental Evolution of accelerated development in Drosophila. 2. Adult fitness and the fast development syndrome. In M. R. Rose, H. B. Passananti & M. Matos (eds.), Methuselah flies: A case study in the evolution of aging (pp. 413–435). Singapore: World Scientific Press.
Chippindale, A. K., Ngo, A. L., & Rose, M. R. (2003). The devil in the details of life-history evolution: Instability and reversal of genetic correlations during selection on Drosophila development. Journal of Genetics, 82, 133–145.
Conner, J. C., & Hartl, D. L. (2004). A primer of ecological genetics. Sunderland: Sinauer Associates Incorporated.
Davidowitz, G., & Nijhout, H. F. (2004). The physiological basis of reaction norms: The interaction between growth rate, the duration of growth and body size. Integrative and Comparative Biology, 44, 443–449.
Del Pino, F., Jara, C., Pino, L., & Godoy-Herrera, R. (2014). The neuro-ecology of Drosophila pupation behavior. PLoS ONE, 9(7), e102159.
Del Pino, F., Salgado, E., & Godoy-Herrera, R. (2012). Plasticity and genotype x environment interactions for locomotion of Drosophila melanogaster larvae. Behavior Genetics, 42, 162–169.
DeWitt, J., & Scheiner, S. M. (2004). Phenotypic plasticity: Functional and conceptual approaches. Oxford: Oxford University Press.
Dillon, M., Wang, G., Garrity, P. A., & Huey, R. B. (2009). Thermal preference in Drosophila. Journal of Thermal Biology, 34, 109–119.
Ebenman, B. (1987). Niche differences between age classes and intraspecific competition in age-structured populations. Journal of Theoretical Biology, 124, 25–33.
Edgar, B. A. (2006). How flies get their size: Genetics meets physiology. Nature Reviews Genetics, 7, 907–916.
Falconer, D. S., & Mackay, T. F. C. (1996). Introduction to quantitative genetics (4th ed.). Essex: Longman.
Fallis, L. C., Fanara, J. J., & Morgan, T. J. (2014). Developmental thermal plasticity among Drosophila melanogaster populations. Journal of Evolutionary Biology, 27, 557–564.
Fanara, J. J., Folguera, G., Fernandez Iriarte, P., Mensch, J., & Hasson, E. (2006). Genotype by environment interactions in viability and developmental time in populations of cactophilic Drosophila. Journal of Evolutionary Biology, 19, 900–906.
Fanara, J. J., & Hasson, E. (2001). Oviposition acceptance and fecundity schedule in the cactophilic sibling species Drosophila buzzatii. and D. koepferae on their natural hosts. Evolution, 55, 2615–2619.
Flatt, T. (2005). The evolutionary genetics of canalization. The Quarterly Review of Biology, 80, 287–316.
Flatt, T., & Heyland, A. (2011). Mechanisms of life history evolution: The genetics and physiology of life history traits and trade-offs. Oxford: Oxford University Press.
Folguera, G., Mensch, J., Muñoz, J. L., Ceballos, S. G., Hasson, E., & Bozinovic, F. (2010). Ontogenetic stage-dependent effect of temperature on developmental and metabolic rates in a holometabolous insect. Journal of Insect Physiology, 56, 1679–1684.
Gerstein, M. B., Rozowsky, J., Yan, K. K., Wang, D., Cheng, C., et al. (2014). Comparative analysis of the transcriptome across distant species. Nature, 512, 445–448.
Gilchrist, G. W., Jeffers, L. M., West, B., Folk, D. G., Suess, J., & Huey, R. B. (2008). Clinal patterns of desiccation and starvation resistance in ancestral and invading populations of Drosophila subobscura. Evolutionary Applications, 1, 513–523.
Goldstein, D. B., & Pollock, D. D. (1997). Launching microsatellites: A review of mutation processes and methods of phylogenetic inference. Journal of Heredity, 88, 335–342.
Hansen, T. F. (2006). The evolution of genetic architecture. Annual Review of Ecology, Evolution, and Systematics, 37, 123–157.
Houle, D. (1992). Comparing evolvability and variability of quantitative traits. Genetics, 130, 195–204.
Huang, W., Massouras, A., Inoue, Y., Peiffer, J., Ràmia, M., et al. (2014). Natural variation in genome architecture among 205 Drosophila melanogaster genetic reference panel lines. Genome Research, 24, 1193–1208.
Jumbo-Lucioni, P., Ayroles, J. F., Chambers, M. M., Jordan, K. W., Leips, J., Mackay, T. F., & De Luca, M. (2010). Systems genetics analysis of body weight and energy metabolism traits in Drosophila melanogaster. BMC Genomics, 11, 297.
Kingsolver, J. G., & Huey, R. B. (2008). Size, temperature, and fitness: Three rules. Evolutionary Ecology Research, 10, 251–268.
Lavagnino, N., Anholt, R. R. H., & Fanara, J. J. (2008). Variation in genetic architecture of olfactory behavior among wild-derived populations of Drosophila melanogaster. Journal of Evolutionary Biology, 21, 988–996.
Lavagnino, N., & Fanara, J. J. (2016). Changes across development influence visible and cryptic natural variation of Drosophila melanogaster olfactory response. Evolutionary Biology, 43, 96–108.
Long, T. A. F., Pischedda, A., Stewart, A. D., & Rice, W. R. (2009). A cost of sexual attractiveness to high-fitness females. PLoS Biology, 7(12), e1000254.
Lynch, M., & Walsh, B. (1998). Genetics and analysis of quantitative traits (p. 663). Sunderland: Sinauer Associates, Inc.
Mackay, T. F. C. (2001). The genetic architecture of quantitative traits. Annual Review of Genetics, 35, 303–339.
Mackay, T. F. C., Richards, S., Stone, E. A., Barbadilla, A., Ayroles, J. F., et al. (2012). The Drosophila melanogaster genetic reference panel. Nature, 482, 173–178.
Markow, T. A. (1979). A survey of intra- and interspecific variation for pupation height in Drosophila. Behavior Genetics, 9, 209–217.
Matzkin, L. M., Watts, T. D., & Markow, T. A. (2007). Desiccation resistance in four Drosophila species: Sex and population effects. Fly, 1, 268–273.
McMahon, D. P., & Hayward, A. (2016). Why grow up? A perspective on insect strategies to avoid metamorphosis. Ecological Entomology, 41, 505–515.
Melo, D., Porto, A., Cheverud, J. M., & Marroig, G. (2016). Modularity: Genes, development, and evolution. Annual Review of Ecology, Evolution, and Systematics, 47, 463–486.
Mensch, J., Carreira, V. P., Lavagnino, N., Goenaga, J., Folguera, G., Hasson, E., & Fanara, J. J. (2010). State-specifics effects of candidate heterochronic genes on variation in developmental time along an altitudinal cline of Drosophila melanogaster. PLoS ONE, 5(6), e11229.
Mensch, J., Lavagnino, N., Carreira, V. P., Massaldi, A., Hasson, E., & Fanara, J. J. (2008). Identifying candidate genes affecting developmental time in Drosophila melanogaster: Pervasive pleiotropy and gene-by-environment interaction. BMC Developmental Biology, 8, 78.
Minelli, A., Brena, C., Deflorian, G., Maruzzo, D., & Fusco, G. (2006). From embryo to adult—beyond the conventional periodization of arthropod development. Development Genes and Evolution, 216, 373–383.
Minelli, A., & Fusco, G. (2010). Developmental plasticity and the evolution of animal complex life cycles. Philosophical Transactions of the Royal Society B, 365, 631–640.
Mirth, C. K., & Riddiford, L. M. (2007). Size assessment and growth control: How adult size is determined in insects. Bioessays, 29, 344–355.
Moran, N. (1994). Adaptation and constraint in the complex life cycles of animals. Annual Review of Ecology and Systematics, 25, 573–600.
Mousseau, T. A., & Roff, D. A. (1987). Natural selection and the heritability of fitness components. Heredity, 59, 181–197.
Mueller, L. D., & Sweet, V. F. (1986). Density-dependent natural selection in Drosophila: Evolution of pupation height. Evolution, 40, 1354–1356.
Narasimha, S., Kolly, S., Sokolowski, M. B., Kawecki, T. J., & Vijendravarma, R. K. (2015). Prepupal building behavior in Drosophila melanogaster and its evolution under resource and time constraints. PLoS ONE, 10, e0117280.
Nunney, L. (1996). The response to selection for fast larval development in Drosophila melanogaster and its effect on adult weight: An example of a fitness trade-off. Evolution, 50, 1193–1204.
Nunney, L. (2007). Pupal period and adult size in Drosophila melanogaster: A cautionary tale of contrasting correlations between two sexually dimorphic traits. Journal of Evolutionary Biology, 20, 141–151.
Nylin, S., & Gotthard, K. (1998). Plasticity in life-history traits. Annual Review of Entomology, 43, 63–83.
Paranjpe, D. A., Anitha, D., Chandrashekaran, M. K., Joshi, A., & Sharma, V. K. (2005). Possible role of eclosion rhythm in mediating the effects of light-dark environments on pre-adult development in Drosophila melanogaster. BMC Developmental Biology, 5, 5.
Partridge, L., Barrie, B., Fowler, K., & French, V. (1994). Thermal evolution of pre-adult life history traits in Drosophila melanogaster. Journal of Evolutionary Biology, 7, 645–663.
Partridge, L., & Fowler, K. (1992). Direct and correlated response to selection on age at reproduction in Drosophila melanogaster. Evolution, 46, 76–91.
Prasad, N. G., Shakarad, M., Anitha, D., Rajamani, M., & Joshi, A. (2001). Correlated responses to selection for faster development and early reproduction in Drosophila: The evolution of larval traits. Evolution, 55, 1363–1372.
Rewitz, K. F., Yamanaka, N., & O’Connor, M. B. (2013). Chapter one—Developmental checkpoints and feedback circuits time insect maturation. Current Topics in Developmental Biology, 103, 1–33.
Riedl, C. A., Riedl, M., Mackay, T. F., & Sokolowski, M. B. (2007). Genetic and behavioral analysis of natural variation in Drosophila melanogaster pupation position. Fly, 1, 23–32.
Rodrigues, M. A., Martins, N. E., Balancé, L. F., Broom, L. N., Dias, A. J., Fernandes, A. S. D., Rodrigues, F., Sucena, E., & Mirth, C. K. (2015). Drosophila melanogaster larvae make nutritional choices that minimize developmental time. Journal of Insect Physiology, 81, 69–80.
Roff, D. A. (1992). The evolution of life histories: Theory and analysis. New York: Chapman & Hall.
Sasaki, A., & Ellner, S. (1997). Quantitative genetic variance maintained by fluctuating selection with overlapping generations: Variance components and covariances. Evolution, 51, 682–696.
Schlichting, C., & Pigliucci, M. (1998). Phenotypic evolution: A reaction norm perspective. Sunderland: Sinauer Associates Incorporated.
Shingleton, A. W., Das, J., Vinicius, L., & Stern, D. L. (2005). The temporal requirements for insulin signaling during development in Drosophila. PLoS Biology, 3(9), e289.
Singh, B. N., & Pandey, M. B. (1993). Evidence for additive polygenic control of pupation heigh in Drosophila ananassae. Hereditas, 119, 111–116.
Sokolowski, M. B., & Hansell, R. I. (1983). Elucidating the behavioral phenotype of Drosophila melanogaster larvae: Correlations between larval foraging strategies and pupation height. Behavior Genetics, 13, 267–280.
Stearns, S. C. (1989). Trade-offs in life-history evolution. Functional Ecology, 3, 259–268.
Stearns, S. C. (1992). The evolution of life histories. Oxford: Oxford University Press.
Trotta, V., Calboli, F. C., Ziosi, M., Guerra, D., Pezzoli, M. C., David, J. R., & Cavicchi, S. (2006). Thermal plasticity in Drosophila melanogaster: A comparison of geographic populations. BMC Evolutionary Biology, 6, 67.
Truman, J. W., & Riddiford, L. M. (1999). The origins of insect metamorphosis. Nature, 401, 447–452.
van Noordwijk, A. J., & de Jong, G. (1986). Acquisition and allocation of resources: Their influence on variation in life history tactics. American Naturalist, 128, 137–142.
Wagner, G. P., Booth, G., & Bagheri-Chaichian, H. (1997). A population genetic theory of canalization. Evolution, 51(2), 329–347.
Welbergen, P., & Sokolowski, M. (1994). Development time and pupation behavior in the Drosophila melanogaster subgroup (Diptera: Drosophilidae). Journal of Insect Behavior, 7, 263–277.
Werenkraut, V., Hasson, E., Oklander, L., & Fanara, J. J. (2008). A comparative study of competitive ability between two cactophilic species in their natural hosts. Austral Ecology, 33, 663–671.
Yang, A. S. (2001). Modularity, evolvability, and adaptive radiations: A comparison of the hemi- and holometabolous insects. Evolution & Development, 3, 59–72.
Acknowledgements
This work was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica (FONCyT, PICT) and Consejo Nacional de Ciencia y Técnica (CONICET). MAPZ and VEO are recipients of doctoral scholarships from CONICET (Argentina) and JJF is a member of Carrera del Investigador Cientifico of CONICET (Argentina).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Petino Zappala, M.A., Ortiz, V.E. & Fanara, J.J. Study of Natural Genetic Variation in Early Fitness Traits Reveals Decoupling Between Larval and Pupal Developmental Time in Drosophila melanogaster. Evol Biol 45, 437–448 (2018). https://doi.org/10.1007/s11692-018-9461-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-018-9461-z