Abstract
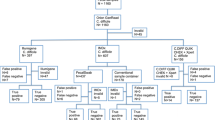
We employed a multiplex polymerase chain reaction (PCR) coupled with capillary electrophoresis (mPCR-CE) targeting six Clostridium difficile genes, including tpi, tcdA, tcdB, cdtA, cdtB, and a deletion in tcdC for simultaneous detection and characterization of toxigenic C. difficile directly from fecal specimens. The mPCR-CE had a limit of detection of 10 colony-forming units per reaction with no cross-reactions with other related bacterial genes. Clinical validation was performed on 354 consecutively collected stool specimens from patients with suspected C. difficile infection and 45 isolates. The results were compared with a reference standard combined with BD MAX Cdiff, real-time cell analysis assay (RTCA), and mPCR-CE. The toxigenic C. difficile species were detected in 36 isolates and 45 stool specimens by the mPCR-CE, which provided a positive rate of 20.3% (81/399). The mPCR-CE had a specificity of 97.2% and a sensitivity of 96.0%, which was higher than RTCA (x2 = 5.67, P = 0.017) but lower than BD MAX Cdiff (P = 0.245). Among the 45 strains, 44 (97.8%) were determined as nonribotype 027 by the mPCR-CE, which was fully agreed with PCR ribotyping. Even though ribotypes 017 (n = 8, 17.8%), 001 (n = 6, 13.3%), and 012 (n = 7, 15.6%) were predominant in this region, ribotype 027 was an important genotype monitored routinely. The mPCR-CE provided an alternative diagnosis tool for the simultaneous detection of toxigenic C. difficile in stool and potentially differentiated between RT027 and non-RT027.
Similar content being viewed by others
References
Leffler DA, Lamont JT. Clostridium difficile Infection. N Engl J Med 2015; 373(3): 287–288
Kelly CP, La Mont JT. Clostridium difficile—more difficult than ever. N Engl J Med 2008; 359(18): 1932–1940
Indra A, Schmid D, Huhulescu S, Hell M, Gattringer R, Hasenberger P, Fiedler A, Wewalka G, Allerberger F. Characterization of clinical Clostridium difficile isolates by PCR ribotyping and detection of toxin genes in Austria, 2006–2007. J Med Microbiol 2008; 57(6): 702–708
McDonald LC, Killgore GE, Thompson A, Owens RC Jr, Kazakova SV, Sambol SP, Johnson S, Gerding DN. An epidemic, toxin genevariant strain of Clostridium difficile. N Engl J Med 2005; 353(23): 2433–2441
Collins DA, Hawkey PM, Riley TV. Epidemiology of Clostridium difficile infection in Asia. Antimicrob Resist Infect Control 2013; 2(1): 21
Asensio A, Monge D. Epidemiology of Clostridium difficile infection in Spain. Enferm Infecc Microbiol Clin 2012; 30(6): 333–337 (in Spanish)
Bourgault AM, Lamothe F, Loo VG, Poirier L. In vitro susceptibility of Clostridium difficile clinical isolates from a multiinstitutional outbreak in Southern Québec, Canada. Antimicrob Agents Chemother 2006; 50(10): 3473–3475
Borgmann S, Kist M, Jakobiak T, Reil M, Scholz E, von Eichel-Streiber C, Gruber H, Brazier JS, Schulte B. Increased number of Clostridium difficile infections and prevalence of Clostridium difficile PCR ribotype 001 in southern Germany. Euro Surveill 2008; 13(49): 19057
Jin D, Luo Y, Huang C, Cai J, Ye J, Zheng Y, Wang L, Zhao P, Liu A, Fang W, Wang X, Xia S, Jiang J, Tang YW. Molecular epidemiology of Clostridium difficile infection in hospitalized patients in Eastern China. J Clin Microbiol 2017;55(3):801–810
Snydman DR, McDermott LA, Jacobus NV, Thorpe C, Stone S, Jenkins SG, Goldstein EJ, Patel R, Forbes BA, Mirrett S, Johnson S, Gerding DN. U.S.-based national sentinel surveillance study for the epidemiology of Clostridium difficile-associated diarrheal isolates and their susceptibility to fidaxomicin. Antimicrob Agents Chemother 2015; 59(10): 6437–6443
Debast SB, Bauer MP, Kuijper EJ; European Society of Clinical Microbiology and Infectious Diseases. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect 2014; 20(Suppl 2): 1–26
Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S, Bourgault AM, Nguyen T, Frenette C, Kelly M, Vibien A, Brassard P, Fenn S, Dewar K, Hudson TJ, Horn R, René P, Monczak Y, Dascal A. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med 2005; 353(23): 2442–2449
Merrigan M, Venugopal A, Mallozzi M, Roxas B, Viswanathan VK, Johnson S, Gerding DN, Vedantam G. Human hypervirulent Clostridium difficile strains exhibit increased sporulation as well as robust toxin production. J Bacteriol 2010; 192(19): 4904–4911
Perelle S, Gibert M, Bourlioux P, Corthier G, Popoff MR. Production of a complete binary toxin (actin-specific ADPribosyltransferase) by Clostridium difficile CD196. Infect Immun 1997; 65(4): 1402–1407
Samie A, Obi CL, Franasiak J, Archbald-Pannone L, Bessong PO, Alcantara-Warren C, Guerrant RL. PCR detection of Clostridium difficile triose phosphate isomerase (tpi), toxin A (tcdA), toxin B (tcdB), binary toxin (cdtA, cdtB), and tcdC genes in Vhembe District, South Africa. Am J Trop Med Hyg 2008; 78(4): 577–585
Lemee L, Dhalluin A, Testelin S, Mattrat MA, Maillard K, Lemeland JF, Pons JL. Multiplex PCR targeting tpi (triose phosphate isomerase), tcdA (Toxin A), and tcdB (Toxin B) genes for toxigenic culture of Clostridium difficile. J Clin Microbiol 2004; 42(12): 5710–5714
Curry SR, Marsh JW, Muto CA, O’Leary MM, Pasculle AW, Harrison LH. tcdC genotypes associated with severe TcdC truncation in an epidemic clone and other strains of Clostridium difficile. J Clin Microbiol 2007; 45(1): 215–221
Brecher SM, Novak-Weekley SM, Nagy E. Laboratory diagnosis of Clostridium difficile infections: there is light at the end of the colon. Clin Infect Dis 2013; 57(8): 1175–1181
Ryder AB, Huang Y, Li H, Zheng M, Wang X, Stratton CW, Xu X, Tang YW. Assessment of Clostridium difficile infections by quantitative detection of tcdB toxin by use of a real-time cell analysis system. J Clin Microbiol 2010; 48(11): 4129–4134
Quinn CD, Sefers SE, Babiker W, He Y, Alcabasa R, Stratton CW, Carroll KC, Tang YW. Diff Quik Chek complete enzyme immunoassay provides a reliable first-line method for detection of Clostridium difficile in stool specimens. J Clin Microbiol 2010; 48 (2): 603–605
Sloan LM, Duresko BJ, Gustafson DR, Rosenblatt JE. Comparison of real-time PCR for detection of the tcdC gene with four toxin immunoassays and culture in diagnosis of Clostridium difficile infection. J Clin Microbiol 2008; 46(6): 1996–2001
Persson S, Torpdahl M, Olsen KE. New multiplex PCR method for the detection of Clostridium difficile toxin A (tcdA) and toxin B (tcdB) and the binary toxin (cdtA/cdtB) genes applied to a Danish strain collection. Clin Microbiol Infect 2008; 14(11): 1057–1064
O’Horo JC, Jones A, Sternke M, Harper C, Safdar N. Molecular techniques for diagnosis of Clostridium difficile infection: systematic review and meta-analysis. Mayo Clin Proc 2012; 87(7): 643–651
Chapin KC, Dickenson RA, Wu F, Andrea SB. Comparison of five assays for detection of Clostridium difficile toxin. J Mol Diagn 2011; 13(4): 395–400
Huang B, Li H, Jin D, Stratton CW, Tang YW. Real-time cellular analysis for quantitative detection of functional Clostridium difficile toxin in stool. Expert Rev Mol Diagn 2014; 14(3): 281–291
Chow WH, Mc Closkey C, Tong Y, Hu L, You Q, Kelly CP, Kong H, Tang YW, Tang W. Application of isothermal helicasedependent amplification with a disposable detection device in a simple sensitive stool test for toxigenic Clostridium difficile. J Mol Diagn 2008; 10(5): 452–458
Polage CR, Gyorke CE, Kennedy MA, Leslie JL, Chin DL, Wang S, Nguyen HH, Huang B, Tang YW, Lee LW, Kim K, Taylor S, Romano PS, Panacek EA, Goodell PB, Solnick JV, Cohen SH. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med 2015; 175(11): 1792–1801
Shin BM, Yoo SM, Shin WC. Evaluation of Xpert C. difficile, BD MAX Cdiff, IMDx C. difficile for Abbott m2000, and Illumigene C. difficile assays for direct detection of toxigenic Clostridium difficile in stool specimens. Ann Lab Med 2016; 36(2): 131–137
Du P, Cao B, Wang J, Li W, Jia H, Zhang W, Lu J, Li Z, Yu H, Chen C, Cheng Y. Sequence variation in tcdA and tcdB of Clostridium difficile: ST37 with truncated tcdA is a potential epidemic strain in China. J Clin Microbiol 2014; 52(9): 3264–3270
Cheng JW, Xiao M, Kudinha T, Xu ZP, Hou X, Sun LY, Zhang L, Fan X, Kong F, Xu YC. The first two Clostridium difficile ribotype 027/ST1 isolates identified in Beijing, China—an emerging problem or a neglected threat? Sci Rep 2016; 6(1): 18834
Wang P, Zhou Y, Wang Z, Xie S, Zhang T, Lin M, Li R, Tan J, Chen Y, Jiang B. Identification of Clostridium difficile ribotype 027 for the first time in Mainland China. Infect Control Hosp Epidemiol 2014; 35(1): 95–98
Oh MH, Paek SH, Shin GW, Kim HY, Jung GY, Oh S. Simultaneous identification of seven foodborne pathogens and Escherichia coli (pathogenic and nonpathogenic) using capillary electrophoresis-based single-strand conformation polymorphism coupled with multiplex PCR. J Food Prot 2009; 72(6): 1262–1266
Hung CC, Chen CP, Lin SP, Chien SC, Lee CN, Cheng WF, Hsieh WS, Liu MS, Su YN, Lin WL. Quantitative assay of deletion or duplication genotype by capillary electrophoresis system: application in Prader–Willi syndrome and Duchenne muscular dystrophy. Clin Chem 2006; 52(12): 2203–2210
Guo L, Qiu B, Chi Y, Chen G. Using multiple PCR and CE with chemiluminescence detection for simultaneous qualitative and quantitative analysis of genetically modified organism. Electrophoresis 2008; 29(18): 3801–3809
Brooks G, Carroll K, Butel J, Morse S. Jawetz, Melnick & Adelberg’s Medical Microbiology. Columbus, OH: McGraw-Hill, 2007
Fang WJ, Jing DZ, Luo Y, Fu CY, Zhao P, Qian J, Tian BR, Chen XG, Zheng YL, Zheng Y, Deng J, Zou WH, Feng XR, Liu FL, Mou XZ, Zheng SS. Clostridium difficile carriage in hospitalized cancer patients: a prospective investigation in Eastern China. BMC Infect Dis 2014; 14(1): 523
Bélanger SD, Boissinot M, Clairoux N, Picard FJ, Bergeron MG. Rapid detection of Clostridium difficile in feces by real-time PCR. J Clin Microbiol 2003; 41(2): 730–734
Sanchez-Vega B, Vega F, Medeiros LJ, Lee MS, Luthra R. Quantification of bcl-2/JH fusion sequences and a control gene by multiplex real-time PCR coupled with automated amplicon sizing by capillary electrophoresis. J Mol Diagn 2002, 4(4): 223–229
Spigaglia P, Mastrantonio P. Molecular analysis of the pathogenicity locus and polymorphism in the putative negative regulator of toxin production (TcdC) among Clostridium difficile clinical isolates. J Clin Microbiol 2002; 40(9): 3470–3475
Huang B, Jin D, Zhang J, Sun JY, Wang X, Stiles J, Xu X, Kamboj M, Babady NE, Tang YW. Real-time cellular analysis coupled with a specimen enrichment accurately detects and quantifies Clostridium difficile toxins in stool. J Clin Microbiol 2014; 52(4): 1105–1111
Indra A, Blaschitz M, Kernbichler S, Reischl U, Wewalka G, Allerberger F. Mechanisms behind variation in the Clostridium difficile 16S-23S rRNA intergenic spacer region. J Med Microbiol 2010; 59(11): 1317–1323
Indra A, Huhulescu S, Schneeweis M, Hasenberger P, Kernbichler S, Fiedler A, Wewalka G, Allerberger F, Kuijper EJ. Characterization of Clostridium difficile isolates using capillary gel electrophoresis-based PCR ribotyping. J Med Microbiol 2008; 57(11): 1377–1382
Carroll KC, Buchan BW, Tan S, Stamper PD, Riebe KM, Pancholi P, Kelly C, Rao A, Fader R, Cavagnolo R, Watson W, Goering RV, Trevino EA, Weissfeld AS, Ledeboer NA. Multicenter evaluation of the Verigene Clostridium difficile nucleic acid assay. J Clin Microbiol 2013; 51(12): 4120–4125
Knight DR, Elliott B, Chang BJ, Perkins TT, Riley TV. Diversity and evolution in the genome of Clostridium difficile. Clin Microbiol Rev 2015; 28(3): 721–741
Yan Q, Zhang J, Chen C, Zhou H, Du P, Cui Z, Cen R, Liu L, Li W, Cao B, Lu J, Cheng Y. Multilocus sequence typing (MLST) analysis of 104 Clostridium difficile strains isolated from China. Epidemiol Infect 2013; 141(1): 195–199
Chen YB, Gu SL, Wei ZQ, Shen P, Kong HS, Yang Q, Li LJ. Molecular epidemiology of Clostridium difficile in a tertiary hospital of China. J Med Microbiol 2014; 63(Pt 4): 562–569
Huang H, Wu S, Chen R, Xu S, Fang H, Weintraub A, Nord CE. Risk factors of Clostridium difficile infections among patients in a university hospital in Shanghai, China. Anaerobe 2014; 30: 65–69
Lihua Z, Danfeng D, Cen J, Xuefeng W, Yibing P. Clinical characterization and risk factors of Clostridium difficile infection in elderly patients in a Chinese hospital. J Infect Dev Ctries 2015; 9(4): 381–387
Wang P, Zhou Y, Wang Z, Xie S, Zhang T, Lin M, Li R, Tan J, Chen Y, Jiang B. Identification of Clostridium difficile ribotype 027 for the first time in Mainland China. Infect Control Hosp Epidemiol 2014; 35(1): 95–98
Deak E, Miller SA, Humphries RM. Comparison of Illumigene, Simplexa, and AmpliVue Clostridium difficile molecular assays for diagnosis of C. difficile infection. J Clin Microbiol 2014; 52(3): 960–963
Spina A, Kerr KG, Cormican M, Barbut F, Eigentler A, Zerva L, Tassios P, Popescu GA, Rafila A, Eerola E, Batista J, Maass M, Aschbacher R, Olsen KE, Allerberger F. Spectrum of enteropathogens detected by the Film Array GI Panel in a multicentre study of community-acquired gastroenteritis. Clin Microbiol Infect 2015; 21 (8): 719–728
Pallis A, Jazayeri J, Ward P, Dimovski K, Svobodova S. Rapid detection of Clostridium difficile toxins from stool samples using real-time multiplex PCR. J Med Microbiol 2013; 62(Pt 9): 1350–1356
Jensen MB, Olsen KE, Nielsen XC, Hoegh AM, Dessau RB, Atlung T, Engberg J. Diagnosis of Clostridium difficile: real-time PCR detection of toxin genes in faecal samples is more sensitive compared to toxigenic culture. Eur J Clin Microbiol Infect Dis 2015; 34(4): 727–736
Guilbault C, Labbé AC, Poirier L, Busque L, Béliveau C, Laverdière M. Development and evaluation of a PCR method for detection of the Clostridium difficile toxin B gene in stool specimens. J Clin Microbiol 2002; 40(6): 2288–2290
Gilbreath JJ, Verma P, Abbott AN, Butler-Wu SM. Comparison of the Verigene Clostridium difficile, Simplexa C. difficile Universal Direct, BD MAX Cdiff, and Xpert C. difficile assays for the detection of toxigenic C. difficile. Diagn Microbiol Infect Dis 2014; 80(1): 13–18
Hirvonen JJ, Kaukoranta SS. Comparison of BD Max Cdiff and GenomEra C. difficile molecular assays for detection of toxigenic Clostridium difficile from stools in conventional sample containers and in FecalSwabs. Eur J Clin Microbiol Infect Dis 2015; 34(5): 1005–1009
Persson S, Jensen JN, Olsen KE. Multiplex PCR method for detection of Clostridium difficile tcdA, tcdB, cdtA, and cdtB and internal in-frame deletion of tcdC. J Clin Microbiol 2011; 49(12): 4299–4300
Spigaglia P. Recent advances in the understanding of antibiotic resistance in Clostridium difficile infection. Ther Adv Infect Dis 2016; 3(1): 23–42
Spigaglia P, Barbanti F, Mastrantonio P. Tetracycline resistance gene tet(W) in the pathogenic bacterium Clostridium difficile. Antimicrob Agents Chemother 2008; 52(2): 770–773
Spigaglia P, Barbanti F, Mastrantonio P, Brazier JS, Barbut F, Delmée M, Kuijper E, Poxton IR; European Study Group on Clostridium difficile (ESGCD). Fluoroquinolone resistance in Clostridium difficile isolates from a prospective study of C. difficile infections in Europe. J Med Microbiol 2008; 57(Pt 6): 784–789
Jalal H, Stephen H, Curran MD, Burton J, Bradley M, Carne C. Development and validation of a rotor-gene real-time PCR assay for detection, identification, and quantification of Chlamydia trachomatis in a single reaction. J Clin Microbiol 2006; 44(1): 206–213
Jin DZ, Wen SY, Chen SH, Lin F, Wang SQ. Detection and identification of intestinal pathogens in clinical specimens using DNA microarrays. Mol Cell Probes 2006; 20(6): 337–347
Tringe SG, Hugenholtz P. A renaissance for the pioneering 16S rRNA gene. Curr Opin Microbiol 2008; 11(5): 442–446
Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, Mc Donald LC, Pepin J, Wilcox MH; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol 2010;31(5):431–455
Acknowledgements
This work was supported by the Programs for Major Science and Technology Medicine and Healthcare in Zhejiang (No. WKJ-ZJ-1507), the Key Research and Development Program of Zhejiang (Nos. 2015G03048 and 2017C03028), the National Natural Science Foundation of China (No. 81471998), the Project of Medical Health and Technology in Zhejiang (No. 2016DTB005), and the National Cancer Institute at the National Institutes of Health Support Grant (No. P30 CA008748).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lai, H., Huang, C., Cai, J. et al. Simultaneous detection and characterization of toxigenic Clostridium difficile directly from clinical stool specimens. Front. Med. 12, 196–205 (2018). https://doi.org/10.1007/s11684-017-0560-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11684-017-0560-5