Abstract
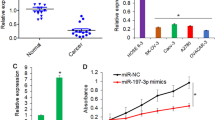
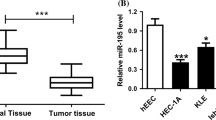
MicroRNAs (miRNAs) play critical roles in the development and progression in various cancers. Dysfunctional miR-9 expression remains ambiguous, and no consensus on the metastatic progression of ovarian cancer has been reached. In this study, results from the bioinformatics analysis show that the 3′-UTR of the E-cadherin mRNA was directly regulated by miR-9. Luciferase reporter assay results confirmed that miR-9 could directly target this 3′-UTR. miR-9 and E-cadherin expression in ovarian cancer tissue was quantified by qRT-PCR. Migration and invasion were detected by wound healing and Transwell system assay in SKOV3 and A2780. qRT-PCR and Western blot were performed to detect the epithelial‒mesenchymal transition-associated mRNA and proteins. Immunofluorescence technique was used to analyze the expression and subcellular localization of E-cadherin, N-cadherin, and vimentin. The results showed that miR-9 was frequently upregulated in metastatic serous ovarian cancer tissue compared with paired primary ones. Upregulation of miR-9 could downregulate the expression of E-cadherin but upregulate the expression of mesenchymal markers (N-cadherin and vimentin). Overexpression of miR-9 could promote the cell migration and invasion in ovarian cancer, and these processes could be effectively inhibited via miR-9 inhibitor. Thus, our study demonstrates that miR-9 may promote ovarian cancer metastasis via targeting E-cadherin and a novel potential therapeutic approach to control metastasis of ovarian cancer.
Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63(1): 11–30
Landen CN, Birrer MJ, Sood AK. Early events in the pathogenesis of epithelial ovarian cancer. J Clin Oncol 2008; 26(6): 995–1005
Cho KR, Shih Ie M. Ovarian cancer. Annu Rev Pathol 2009; 4(1): 287–313
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116(2): 281–297
Slack FJ, Weidhaas JB. MicroRNA in cancer prognosis. N Engl J Med 2008; 359(25): 2720–2722
Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell 2005; 122(1): 6–7
Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science 2004; 303(5654): 83–86
Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D, Teruya- Feldstein J, Reinhardt F, Onder TT, Valastyan S, Westermann F, Speleman F, Vandesompele J, Weinberg RA. miR-9, a MYC/ MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol 2010; 12(3): 247–256
Song Y, Li J, Zhu Y, Dai Y, Zeng T, Liu L, Li J, Wang H, Qin Y, Zeng M, Guan XY, Li Y. MicroRNA-9 promotes tumor metastasis via repressing E-cadherin in esophageal squamous cell carcinoma. Oncotarget 2014; 5(22): 11669–11680
Sun Z, Han Q, Zhou N,Wang S, Lu S, Bai C, Zhao RC. MicroRNA-9 enhances migration and invasion through KLF17 in hepatocellular carcinoma. Mol Oncol 2013; 7(5): 884–894
Zhu L, Chen H, Zhou D, Li D, Bai R, Zheng S, Ge W. MicroRNA-9 up-regulation is involved in colorectal cancer metastasis via promoting cell motility. Med Oncol 2012; 29(2): 1037–1043
Shiiyama R, Fukushima S, Jinnin M, Yamashita J, Miyashita A, Nakahara S, Kogi A, Aoi J, Masuguchi S, Inoue Y, Ihn H. Sensitive detection of melanoma metastasis using circulating microRNA expression profiles. Melanoma Res 2013; 23(5): 366–372
Iorio MV, Visone R, Di Leva G, Donati V, Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, Calin GA, Menard S, Croce CM. MicroRNA signatures in human ovarian cancer. Cancer Res 2007; 67(18): 8699–8707
Laios A, O’Toole S, Flavin R, Martin C, Kelly L, Ring M, Finn SP, Barrett C, Loda M, Gleeson N, D’Arcy T, McGuinness E, Sheils O, Sheppard B, O’ Leary J. Potential role of miR-9 and miR-223 in recurrent ovarian cancer. Mol Cancer 2008; 7(1): 35
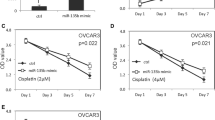
Sun C, Li N, Yang Z, Zhou B, He Y, Weng D, Fang Y, Wu P, Chen P, Yang X, Ma D, Zhou J, Chen G. miR-9 regulation of BRCA1 and ovarian cancer sensitivity to cisplatin and PARP inhibition. J Natl Cancer Inst 2013; 105(22): 1750–1758
Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002; 2(6): 442–454
Dai Y, Zhou X. Computational methods for the identification of microRNA targets. Open Access Bioinformatics 2010; 2:29–39
Dweep H, Sticht C, Pandey P, Gretz N. miRWalk–database: prediction of possible miRNA binding sites by “walking” the genes of three genomes. J Biomed Inform 2011; 44(5): 839–847
Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2007; 2(2): 329–333
Valster A, Tran NL, Nakada M, Berens ME, Chan AY, Symons M. Cell migration and invasion assays. Methods 2005; 37(2): 208–215
Weng D, Song X, Xing H, Ma X, Xia X, Weng Y, Zhou J, Xu G, Meng L, Zhu T, Wang S, Ma D. Implication of the Akt2/survivin pathway as a critical target in paclitaxel treatment in human ovarian cancer cells. Cancer Lett 2009; 273(2): 257–265
Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelialmesenchymal transitions in development and disease. Cell 2009; 139(5): 871–890
Vergara D, Merlot B, Lucot JP, Collinet P, Vinatier D, Fournier I, Salzet M. Epithelial-mesenchymal transition in ovarian cancer. Cancer Lett 2010; 291(1): 59–66
Hurst DR, Edmonds MD, Welch DR. MetastamiR: the field of metastasis-regulatory microRNA is spreading. Cancer Res 2009; 69(19): 7495–7498
Deo M, Yu JY, Chung KH, Tippens M, Turner DL. Detection of mammalian microRNA expression by in situ hybridization with RNA oligonucleotides. Dev Dyn 2006; 235(9): 2538–2548
Nass D, Rosenwald S, Meiri E, Gilad S, Tabibian-Keissar H, Schlosberg A, Kuker H, Sion-Vardy N, Tobar A, Kharenko O, Sitbon E, Lithwick Yanai G, Elyakim E, Cholakh H, Gibori H, Spector Y, Bentwich Z, Barshack I, Rosenfeld N. miR-92b and miR-9/9* are specifically expressed in brain primary tumors and can be used to differentiate primary from metastatic brain tumors. Brain Pathol 2009; 19(3): 375–383
Luo X, Fan S, Huang W, Zhai S, Ma Z, Li P, Sun SY, Wang X. Downregulation of IRS-1 promotes metastasis of head and neck squamous cell carcinoma. Oncol Rep 2012; 28(2): 659–667
Lu MH, Huang CC, Pan MR, Chen HH, Hung WC. Prospero homeobox 1 promotes epithelial-mesenchymal transition in colon cancer cells by inhibiting E-cadherin via miR-9. Clin Cancer Res 2012; 18(23): 6416–6425
Gwak JM, Kim HJ, Kim EJ, Chung YR, Yun S, Seo AN, Lee HJ, Park SY. MicroRNA-9 is associated with epithelial-mesenchymal transition, breast cancer stem cell phenotype, and tumor progression in breast cancer. Breast Cancer Res Treat 2014; 147(1): 39–49
Wilting SM, Snijders PJ, Verlaat W, Jaspers A, van de Wiel MA, van Wieringen WN, Meijer GA, Kenter GG, Yi Y, le Sage C, Agami R, Meijer CJ, Steenbergen RD. Altered microRNA expression associated with chromosomal changes contributes to cervical carcinogenesis. Oncogene 2013; 32(1): 106–116
Zheng L, Qi T, Yang D, Qi M, Li D, Xiang X, Huang K, Tong Q. MicroRNA-9 suppresses the proliferation, invasion and metastasis of gastric cancer cells through targeting cyclin D1 and Ets1. PLoS One 2013; 8(1): e55719
Omura N, Li CP, Li A, Hong SM, Walter K, Jimeno A, Hidalgo M, Goggins M. Genome-wide profiling of methylated promoters in pancreatic adenocarcinoma. Cancer Biol Ther 2008; 7(7): 1146–1156
Lehmann U, Hasemeier B, Christgen M, Muller M, Romermann D, Langer F, Kreipe H. Epigenetic inactivation of microRNA gene hsamir- 9–1 in human breast cancer. J Pathol 2008; 214(1): 17–24
Inoue T, Iinuma H, Ogawa E, Inaba T, Fukushima R. Clinicopathological and prognostic significance of microRNA-107 and its relationship to DICER1 mRNA expression in gastric cancer. Oncol Rep 2012; 27(6): 1759–1764
Qiu Y, Luo X, Kan T, Zhang Y, Yu W, Wei Y, Shen N, Yi B, Jiang X. TGF-ß upregulates miR-182 expression to promote gallbladder cancer metastasis by targeting CADM1. Mol Biosyst 2014; 10(3): 679–685
Yu J, Lei R, Zhuang X, Li X, Li G, Lev S, Segura MF, Zhang X, Hu G. MicroRNA-182 targets SMAD7 to potentiate TGFß-induced epithelial-mesenchymal transition and metastasis of cancer cells. Nat Commun 2016; 7: 13884
Acknowledgements
This work was supported by the National Basic Research Program of China (973 Program, No. 2015CB553903), National Natural Science Foundation of China (Nos. 81272859, 81372801, 81230038, 81272422, 81302266, 81402163, 81402164, 81501530, and 81572569), and the Science and Technology Project of Shenzhen (No. Jcyj20140416122811911).
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhou, B., Xu, H., Xia, M. et al. Overexpressed miR-9 promotes tumor metastasis via targeting E-cadherin in serous ovarian cancer. Front. Med. 11, 214–222 (2017). https://doi.org/10.1007/s11684-017-0518-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11684-017-0518-7