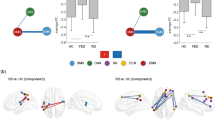
Abstract
Brain imaging reveals schizophrenia as a disorder of macroscopic brain networks. In particular, default mode and salience network (DMN, SN) show highly consistent alterations in both interacting brain activity and underlying brain structure. However, the same networks are also altered in major depression. This overlap in network alterations induces the question whether DMN and SN changes are different across both disorders, potentially indicating distinct underlying pathophysiological mechanisms. To address this question, we acquired T1-weighted, diffusion-weighted, and resting-state functional MRI in patients with schizophrenia, patients with major depression, and healthy controls. We measured regional gray matter volume, inter-regional structural and intrinsic functional connectivity of DMN and SN, and compared these measures across groups by generalized Wilcoxon rank tests, while controlling for symptoms and medication. When comparing patients with controls, we found in each patient group SN volume loss, impaired DMN structural connectivity, and aberrant DMN and SN functional connectivity. When comparing patient groups, SN gray matter volume loss and DMN structural connectivity reduction did not differ between groups, but in schizophrenic patients, functional hyperconnectivity between DMN and SN was less in comparison to depressed patients. Results provide evidence for distinct functional hyperconnectivity between DMN and SN in schizophrenia and major depression, while structural changes in DMN and SN were similar. Distinct hyperconnectivity suggests different pathophysiological mechanism underlying aberrant DMN-SN interactions in schizophrenia and depression.



Similar content being viewed by others
References
Abou-Elseoud, A., Starck, T., Remes, J., Nikkinen, J., Tervonen, O., & Kiviniemi, V. (2010). The effect of model order selection in group PICA. Human Brain Mapping, 31(8), 1207–1216.
Allen, E. A., Erhardt, E. B., Damaraju, E., Gruner, W., Segall, J. M., Silva, R. F., et al. (2011). A baseline for the multivariate comparison of resting-state networks. Frontiers in Systems Neuroscience, 5, 2.
American Psychiatric Association. (2000). DSM-IV-TR: Diagnostic and statistical manual of mental disorders, text revision. Washington, DC: American Psychiatric Association, 75.
Biswal, B., Zerrin Yetkin, F., Haughton, V. M., & Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magnetic Resonance in Medicine, 34(4), 537–541.
Bora, E., Fornito, A., Pantelis, C., & Yücel, M. (2012). Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. Journal of Affective Disorders, 138(1), 9–18.
Borgwardt, S. J., McGUIRE, P. K., Aston, J., Berger, G., Dazzan, P., Gschwandtner, U. T. E., et al. (2007). Structural brain abnormalities in individuals with an at-risk mental state who later develop psychosis. The British Journal of Psychiatry, 191(51), s69-s75.
Bragulat, V., Paillère-Martinot, M. L., Artiges, E., Frouin, V., Poline, J. B., & Martinot, J. L. (2007). Dopaminergic function in depressed patients with affective flattening or with impulsivity:[18 F] fluoro-L-dopa positron emission tomography study with voxel-based analysis. Psychiatry Research: Neuroimaging, 154(2), 115–124.
Brunner, E., & Munzel, U. (2000). The nonparametric Behrens-Fisher problem: asymptotic theory and a small-sample approximation. Biometrical Journal, 42(1), 17–25.
Buchsbaum, M. S., Schoenknecht, P., Torosjan, Y., Newmark, R., Chu, K. W., Mitelman, S., et al. (2006). Diffusion tensor imaging of frontal lobe white matter tracts in schizophrenia. Annals of General Psychiatry, 5(1), 19.
Chen, X., Duan, M., Xie, Q., Lai, Y., Dong, L., Cao, W., et al. (2015). Functional disconnection between the visual cortex and the sensorimotor cortex suggests a potential mechanism for self-disorder in schizophrenia. Schizophrenia Research, 166(1), 151–157.
Cole, D. M., Oei, N. Y., Soeter, R. P., Both, S., van Gerven, J. M., Rombouts, S. A., & Beckmann, C. F. (2013). Dopamine-dependent architecture of cortico-subcortical network connectivity. Cerebral Cortex, 23(7), 1509–1516.
Corbetta, M., & Shulman, G. L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3(3), 201–215.
Deco, G., Jirsa, V. K., & McIntosh, A. R. (2013). Resting brains never rest: computational insights into potential cognitive architectures. Trends in Neurosciences, 36(5), 268–274.
Dong, D., Wang, Y., Chang, X., Luo, C., & Yao, D. (2017). Dysfunction of large-scale brain networks in Schizophrenia: a meta-analysis of resting-state functional connectivity. Schizophrenia bulletin, sbx034.
Duncan, N. W., Wiebking, C., & Northoff, G. (2014). Associations of regional GABA and glutamate with intrinsic and extrinsic neural activity in humans—A review of multimodal imaging studies. Neuroscience & Biobehavioral Reviews, 47, 36–52.
Ellison-Wright, I., & Bullmore, E. (2009). Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophrenia Research, 108(1), 3–10.
Ellison-Wright, I., Glahn, D. C., Laird, A. R., Thelen, S. M., & Bullmore, E. (2008). The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. American Journal of Psychiatry, 165(8), 1015–1023.
Fox, M. D., & Raichle, M. E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience, 8(9), 700–711.
Frodl, T. S., Koutsouleris, N., Bottlender, R., Born, C., Jäger, M., Scupin, I., … Meisenzahl, E. M. (2008). Depression-related variation in brain morphology over 3 years: effects of stress? Archives of General Psychiatry, 65(10), 1156–1165.
Goodkind, M., Eickhoff, S. B., Oathes, D. J., Jiang, Y., Chang, A., Jones-Hagata, L. B., et al. (2015). Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry, 72(4), 305–315.
Greicius, M. D., Flores, B. H., Menon, V., Glover, G. H., Solvason, H. B., Kenna, H., et al. (2007). Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry, 62(5), 429–437.
Greicius, M. D., Krasnow, B., Reiss, A. L., & Menon, V. (2003). Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences, 100(1), 253–258.
Hagmann, P., Cammoun, L., Gigandet, X., Meuli, R., Honey, C. J., Wedeen, V. J., & Sporns, O. (2008). Mapping the structural core of human cerebral cortex. PLoS Biolology, 6(7), e159.
Hahn, K., Myers, N., Prigarin, S., Rodenacker, K., Kurz, A., Förstl, H., et al. (2013). Selectively and progressively disrupted structural connectivity of functional brain networks in Alzheimer’s disease—revealed by a novel framework to analyze edge distributions of networks detecting disruptions with strong statistical evidence. Neuroimage, 81, 96–109.
Hamilton, J. P., Chen, M. C., & Gotlib, I. H. (2013). Neural systems approaches to understanding major depressive disorder: an intrinsic functional organization perspective. Neurobiology of Disease, 52, 4–11.
Hamilton, M. (1960). A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry, 23(1), 56–62.
Howes, O. D., Bose, S. K., Turkheimer, F., Valli, I., Egerton, A., Valmaggia, L. R., et al. (2011). Dopamine synthesis capacity before onset of psychosis: a prospective [18F]-DOPA PET imaging study. American Journal of Psychiatry, 168(12), 1311–1317.
Kaiser, R. H., Andrews-Hanna, J. R., Wager, T. D., & Pizzagalli, D. A. (2015). Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry, 72(6), 603–611.
Kay, S. R., Flszbein, A., & Opfer, L. A. (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin, 13(2), 261.
Kieseppä, T., Eerola, M., Mäntylä, R., Neuvonen, T., Poutanen, V. P., Luoma, K., et al. (2010). Major depressive disorder and white matter abnormalities: a diffusion tensor imaging study with tract-based spatial statistics. Journal of Affective Disorders, 120(1), 240–244.
Kraguljac, N. V., White, D. M., Reid, M. A., & Lahti, A. C. (2013). Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA Psychiatry, 70(12), 1294–1302.
Lazar, M., Weinstein, D. M., Tsuruda, J. S., Hasan, K. M., Arfanakis, K., Meyerand, M. E., et al. (2003). White matter tractography using diffusion tensor deflection. Human Brain Mapping, 18(4), 306–321.
Liao, Y., Huang, X., Wu, Q., Yang, C., Kuang, W., Du, M., et al. (2013). Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. Journal of Psychiatry & Neuroscience: JPN, 38(1), 49.
Luo, C., Zhang, Y., Cao, W., Huang, Y., Yang, F., Wang, J., et al. (2015). Altered structural and functional feature of striato-cortical circuit in benign epilepsy with centrotemporal spikes. International Journal of Neural Systems, 25(06), 1550027.
Manoliu, A., Meng, C., Brandl, F., Doll, A., Tahmasian, M., Scherr, M., et al. (2013a). Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Frontiers in Human Neuroscience, 7, 930. https://doi.org/10.3389/fnhum.2013.00930.
Manoliu, A., Riedl, V., Doll, A., Bäuml, J. G., Mühlau, M., Schwerthöffer, D., et al. (2013b). Insular dysfunction reflects altered between-network connectivity and severity of negative symptoms in schizophrenia during psychotic remission. Frontiers in Human Neuroscience, 7, 216.
Manoliu, A., Riedl, V., Zherdin, A., Mühlau, M., Schwerthöffer, D., Scherr, M., et al. (2014). Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophrenia Bulletin, 40(2), 428–437.
Meng, C., Brandl, F., Tahmasian, M., Shao, J., Manoliu, A., Scherr, M., et al. (2013). Aberrant topology of striatum’s connectivity is associated with the number of episodes in depression. Brain, 137, 598–609.
Menon, V. (2011). Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Sciences, 15(10), 483–506.
Mulders, P. C., van Eijndhoven, P. F., Schene, A. H., Beckmann, C. F., & Tendolkar, I. (2015). Resting-state functional connectivity in major depressive disorder: a review. Neuroscience & Biobehavioral Reviews, 56, 330–344.
Northoff, G., & Sibille, E. (2014). Cortical GABA neurons and self-focus in depression: a model linking cellular, biochemical, and neural network findings. Molecular Psychiatry, 19(9), 959.
Orliac, F., Naveau, M., Joliot, M., Delcroix, N., Razafimandimby, A., Brazo, P., et al. (2013). Links among resting-state default-mode network, salience network, and symptomatology in schizophrenia. Schizophrenia Research, 148(1), 74–80.
Palaniyappan, L., & Liddle, P. F. (2012). Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. Journal of Psychiatry & Neuroscience: JPN, 37, 17–27.
Palaniyappan, L., Simmonite, M., White, T. P., Liddle, E. B., & Liddle, P. F. (2013). Neural primacy of the salience processing system in schizophrenia. Neuron, 79(4), 814–828.
Pessoa, L. (2014). Brain networks: Moving beyond graphs. Reply to comments on ``understanding brain networks and brain organization’’. Physics of Life Reviews, 11, 462–466.
Schmidt, A., Diwadkar, V. A., Smieskova, R., Harrisberger, F., Lang, U. E., McGuire, P., et al. (2014). Approaching a network connectivity-driven classification of the psychosis continuum: a selective review and suggestions for future research. Frontiers in Human Neuroscience, 8, 1047.
Seeley, W. W., Menon, V., Schatzberg, A. F., Keller, J., Glover, G. H., Kenna, H., et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–2356.
Shao, J., Myers, N., Yang, Q., Feng, J., Plant, C., Böhm, C., et al. (2012). Prediction of Alzheimer’s disease using individual structural connectivity networks. Neurobiology of Aging, 33(12), 2756–2765.
Sheline, Y. I., Price, J. L., Yan, Z., & Mintun, M. A. (2010). Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences, 107(24), 11020–11025.
Sorg, C., Manoliu, A., Neufang, S., Myers, N., Peters, H., Schwerthöffer, D., et al. (2013). Increased intrinsic brain activity in the striatum reflects symptom dimensions in schizophrenia. Schizophrenia Bulletin, 39(2), 387–395.
Spitzer, R. L., Williams, J. B., Gibbon, M., & First, M. B. (1992). The structured clinical interview for DSM-III-R (SCID): I: history, rationale, and description. Archives of General Psychiatry, 49(8), 624–629.
Stephan, K. E., Friston, K. J., & Frith, C. D. (2009). Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. SchizophreniaBulletin, 35, 509–527.
Tahmasian, M., Knight, D. C., Manoliu, A., Schwerthöffer, D., Scherr, M., Meng, C., et al. (2013). Aberrant intrinsic connectivity of hippocampus and amygdala overlap in the fronto-insular and dorsomedial-prefrontal cortex in major depressive disorder. Frontiers in Human Neuroscience, 7, 639.
Uddin, L. Q., Supekar, K. S., Ryali, S., & Menon, V. (2011). Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. Journal of Neuroscience, 31(50), 18578–18589.
van den Heuvel, M. P., & Fornito, A. (2014). Brain networks in schizophrenia. Neuropsychology Review, 24(1), 32–48.
Van Dijk, K. R., Sabuncu, M. R., & Buckner, R. L. (2012). The influence of head motion on intrinsic functional connectivity MRI. Neuroimage, 59(1), 431–438.
Walter, M., Henning, A., Grimm, S., Schulte, R. F., Beck, J., Dydak, U., et al. (2009). The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Archives of General Psychiatry, 66(5), 478–486.
Wang, D., Zhou, Y., Zhuo, C., Qin, W., Zhu, J., Liu, H., et al. (2015). Altered functional connectivity of the cingulate subregions in schizophrenia. Translational Psychiatry, 5(6), e575.
Whitfield-Gabrieli, S., Thermenos, H. W., Milanovic, S., Tsuang, M. T., Faraone, S. V., McCarley, R. W., et al. (2009). Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proceedings of the National Academy of Sciences, 106(4), 1279–1284.
Williamson, P. (2007). Are anticorrelated networks in the brain relevant to schizophrenia? Schizophrenia Bulletin, 33(4), 994–1003.
Wotruba, D., Michels, L., Buechler, R., Metzler, S., Theodoridou, A., Gerstenberg, M., et al. (2013). Aberrant coupling within and across the default mode, task-positive, and salience network in subjects at risk for psychosis. Schizophrenia Bulletin, 40, 1095–1104.
Funding
This study was supported by the National Natural Science Foundation of China (61403062, 61433014 to J.S.), China Postdoctoral Science Foundation (2015M580786 to J.S., 2014M552344, 2015T80973 to Q.Y.), Science-Technology Foundation for Young Scientist of SiChuan Province (2016JQ0007 to J.S.) and the German Federal Ministry of Education and Research (BMBF 01EV0710 to A.M.W., BMBF 01ER0803 to C.S.)
Author information
Authors and Affiliations
Contributions
JS and CS designed the study; CM, MT, AM, MS and DS recruited participants and acquired data; JB and HF acquired data; JS, QY, GL, CL, DY and LG analysed data; CZ, VR, AW and CS interpreted data; JS and CS drafted the article; all authors critically revised and approved the final version of the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Shao, J., Meng, C., Tahmasian, M. et al. Common and distinct changes of default mode and salience network in schizophrenia and major depression. Brain Imaging and Behavior 12, 1708–1719 (2018). https://doi.org/10.1007/s11682-018-9838-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-018-9838-8