Abstract
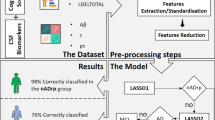
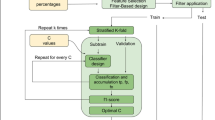
We evaluated the performance of amyloid PET textural and shape features in discriminating normal and Alzheimer’s disease (AD) subjects, and in predicting conversion to AD in subjects with mild cognitive impairment (MCI) or significant memory concern (SMC). Subjects from the Alzheimer’s Disease Neuroimaging Initiative with available baseline 18F-florbetapir and T1-MRI scans were included. The cross-sectional cohort consisted of 181 controls and 148 AD subjects. The longitudinal cohort consisted of 431 SMC/MCI subjects, 85 of whom converted to AD during follow-up. PET images were normalized to MNI space and post-processed using in-house software. Relative retention indices (SUVr) were computed with respect to pontine, cerebellar, and composite reference regions. Several textural and shape features were extracted then combined using a support vector machine (SVM) to build a predictive model of AD conversion. Diagnostic and prognostic performance was evaluated using ROC analysis and survival analysis with the Cox proportional hazard model. The three SUVr and all the tested features effectively discriminated AD subjects in cross-sectional analysis (all p < 0.001). In longitudinal analysis, the variables with the highest prognostic value were composite SUVr (AUC 0.86; accuracy 81%), skewness (0.87; 83%), local minima (0.85; 79%), Geary’s index (0.86; 81%), gradient norm maximal argument (0.83; 82%), and the SVM model (0.91; 86%). The adjusted hazard ratio for AD conversion was 5.5 for the SVM model, compared with 4.0, 2.6, and 3.8 for cerebellar, pontine and composite SUVr (all p < 0.001), indicating that appropriate amyloid textural and shape features predict conversion to AD with at least as good accuracy as classical SUVr.






Similar content being viewed by others
Notes
The software is currently under beta-testing for routine exploitation and will soon be made available for download at: http://scinti.edu.umontpellier.fr/recherche/logiciels-a-telecharger/.
References
Akamatsu, G., Ikari, Y., Ohnishi, A., Nishida, H., Aita, K., Sasaki, M., Yamamoto, Y., Sasaki, M., & Senda, M. (2016). Automated PET-only quantification of amyloid deposition with adaptive template and empirically pre-defined ROI. Physics in Medicine and Biology, 61, 5768–5780.
Apostolova, I., Ego, K., Steffen, I. G., Buchert, R., Wertzel, H., Achenbach, H. J., Riedel, S., Schreiber, J., Schultz, M., Furth, C., Derlin, T., Amthauer, H., Hofheinz, F., & Kalinski, T. (2016). The asphericity of the metabolic tumour volume in NSCLC: correlation with histopathology and molecular markers. European Journal of Nuclear Medicine and Molecular Imaging, 43, 2360–2373.
Ben Bouallègue, F., Al Tabaa, Y., Kafrouni, M., Cartron, G., Vauchot, F., & Mariano-Goulart, D. (2017): Association between textural and morphological tumor indices on baseline PET-CT and early metabolic response on interim PET-CT in bulky malignant lymphomas. Med Phys [Epub ahead of print].
Ben Bouallègue, F., Mariano-Goulart, D., & Payoux, P. Alzheimer’s Disease Neuroimaging Initiative (2017): Comparison of CSF markers and semi-quantitative amyloid PET in Alzheimer’s disease diagnosis and in cognitive impairment prognosis using the ADNI-2 database. Alzheimer’s Research & Therapy 9(1):32.
Boccardi, M., Altomare, D., Ferrari, C., Festari, C., Guerra, U. P., Paghera, B., Pizzocaro, C., Lussignoli, G., Geroldi, C., Zanetti, O., Cotelli, M. S., Turla, M., Borroni, B., Rozzini, L., Mirabile, D., Defanti, C., Gennuso, M., Prelle, A., Gentile, S., Morandi, A., Vollaro, S., Volta, G. D., Bianchetti, A., Conti, M. Z., Cappuccio, M., Carbone, P., Bellandi, D., Abruzzi, L., Bettoni, L., Villani, D., Raimondi, M. C., Lanari, A., Ciccone, A., Facchi, E., Di Fazio, I., Rozzini, R., Boffelli, S., Manzoni, L., Salvi, G. P., Cavaliere, S., Belotti, G., Avanzi, S., Pasqualetti, P., Muscio, C., Padovani, A., & Frisoni, G. B. Incremental Diagnostic Value of Amyloid PET With [18F]-Florbetapir (INDIA-FBP) Working Group (2016): Assessment of the Incremental Diagnostic Value of Florbetapir F 18 Imaging in Patients With Cognitive Impairment: The Incremental Diagnostic Value of Amyloid PET With [18F]-Florbetapir (INDIA-FBP) Study. JAMA Neurology 73:1417–1424.
Brendel, M., Högenauer, M., Delker, A., Sauerbeck, J., Bartenstein, P., Seibyl, J., & Rominger, A. Alzheimer’s Disease Neuroimaging Initiative (2015): Improved longitudinal [(18)F]-AV45 amyloid PET by white matter reference and VOI-based partial volume effect correction. Neuroimage 108:450–459.
Buvat, I., Orlhac, F., & Soussan, M. (2015). Tumor Texture Analysis in PET: Where Do We Stand? Nuclear Medicine, 56, 1642–1644.
Chen, K., Roontiva, A., Thiyyagura, P., Lee, W., Liu, X., Ayutyanont, N., Protas, H., Luo, J. L., Bauer, R., Reschke, C., Bandy, D., Koeppe, R. A., Fleisher, A. S., Caselli, R. J., Landau, S., Jagust, W. J., Weiner, M. W., & Reiman, E. M. Alzheimer’s Disease Neuroimaging Initiative (2015): Improved power for characterizing longitudinal amyloid-β PET changes and evaluating amyloid-modifying treatments with a cerebral white matter reference region. Journal of Nuclear Medicine 56:560–566.
Chicklore, S., Goh, V., Siddique, M., Roy, A., Marsden, P. K., & Cook, G. J. R. (2013). Quantifying tumour heterogeneity in 18F-FDG PET/CT imaging by texture analysis. European Journal of Nuclear Medicine and Molecular Imaging, 40, 133–140.
Chincarini, A., Sensi, F., Rei, L., Bossert, I., Morbelli, S., Guerra, U. P., Frisoni, G., Padovani, A., & Nobili, F. Alzheimer’s Disease Neuroimaging Initiative (2016): Standardized Uptake Value Ratio-Independent Evaluation of Brain Amyloidosis. Journal of Alzheimer’s Disease 54:1437–1457.
Clark, C. M., Pontecorvo, M. J., Beach, T. G., Bedell, B. J., Coleman, R. E., Doraiswamy, P. M., Fleisher, A. S., Reiman, E. M., Sabbagh, M. N., Sadowsky, C. H., Schneider, J. A., Arora, A., Carpenter, A. P., Flitter, M. L., Joshi, A. D., Krautkramer, M. J., Lu, M., Mintun, M. A., & Skovronsky, D. M. AV-45-A16 Study Group (2012): Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol 11:669–678.
Doraiswamy, P. M., Sperling, R. A., Johnson, K., Reiman, E. M., Wong, T. Z., Sabbagh, M. N., Sadowsky, C. H., Fleisher, A. S., Carpenter, A., Joshi, A. D., Lu, M., Grundman, M., Mintun, M. A., Skovronsky, D. M., & Pontecorvo, M. J. AV45-A11 Study Group, AV45-A11 Study Group (2014): Florbetapir F 18 amyloid PET and 36-month cognitive decline: a prospective multicenter study. Molecular Psychiatry 19:1044–1051.
Dubois, B., Feldman, H. H., Jacova, C., Dekosky, S. T., Barberger-Gateau, P., Cummings, J., Delacourte, A., Galasko, D., Gauthier, S., Jicha, G., Meguro, K., O’brien, J., Pasquier, F., Robert, P., Rossor, M., Salloway, S., Stern, Y., Visser, P. J., & Scheltens, P. (2007). Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurology, 6, 734–746.
Dukart, J., Mueller, K., Barthel, H., Villringer, A., Sabri, O., & Schroeter, M. L. Alzheimer’s Disease Neuroimaging Initiative (2013): Meta-analysis based SVM classification enables accurate detection of Alzheimer’s disease across different clinical centers using FDG-PET and MRI. Psychiatry Research 212:230–236.
El Naqa, I., Grigsby, P., Apte, A., Kidd, E., Donnelly, E., Khullar, D., Chaudhari, S., Yang, D., Schmitt, M., Laforest, R., Thorstad, W., & Deasy, J. O. (2009). Exploring feature-based approaches in PET images for predicting cancer treatment outcomes. Pattern Recognition, 42, 1162–1171.
Ellendt, S., Voß, B., Kohn, N., Wagels, L., Goerlich, K., Drexler, E., Schneider, F., & Habel, U. (2016): Predicting stability of Mild Cognitive Impairment (MCI): findings of a community based sample. Curr Alzheimer Res.
Falconer, K. 1990. Fractal geometry: mathematical foundations and applications. John Wiley.
Goh, V., Sanghera, B., Wellsted, D. M., Sundin, J., & Halligan, S. (2009). Assessment of the spatial pattern of colorectal tumour perfusion estimated at perfusion CT using two-dimensional fractal analysis. European Radiology, 19, 1358–1365.
Gonzalez-Escamilla, G., Lange, C., Teipel, S., Buchert, R., & Grothe, M. J. Alzheimer’s Disease Neuroimaging Initiative (2017): PETPVE12: an SPM toolbox for Partial Volume Effects correction in brain PET - Application to amyloid imaging with AV45-PET. Neuroimage 147:669–677.
Haralick, R. M., Shanmugam, K., & Dinstein, I. (1973). Textural features for image classification. IEEE Transactions on Systems, Man, and Cybernetics, 3, 610–621.
Hayano, K., Lee, S. H., Yoshida, H., Zhu, A. X., & Sahani, D. V. (2014). Fractal analysis of CT perfusion images for evaluation of antiangiogenic treatment and survival in hepatocellular carcinoma. Academic Radiology, 21, 654–660.
Hsiao, I. T., Huang, C. C., Hsieh, C. J., Wey, S. P., Kung, M. P., Yen, T. C., & Lin, K. J. (2013). Perfusion-like template and standardized normalization-based brain image analysis using 18F-florbetapir (AV-45/Amyvid) PET. European Journal of Nuclear Medicine and Molecular Imaging, 40, 908–920.
Jack, C. R., Knopman, D. S., Jagust, W. J., Shaw, L. M., Aisen, P. S., Weiner, M. W., Petersen, R. C., & Trojanowski, J. Q. (2010). Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurology, 9, 119–128.
Joshi, A., Koeppe, R. A., & Fessler, J. A. (2009). Reducing between scanner differences in multi-center PET studies. Neuroimage, 46, 154–159.
Joshi, A. D., Pontecorvo, M. J., Clark, C. M., Carpenter, A. P., Jennings, D. L., Sadowsky, C. H., Adler, L. P., Kovnat, K. D., Seibyl, J. P., Arora, A., Saha, K., Burns, J. D., Lowrey, M. J., Mintun, M. A., & Skovronsky, D. M. Florbetapir F 18 Study Investigators (2012): Performance characteristics of amyloid PET with florbetapir F 18 in patients with alzheimer’s disease and cognitively normal subjects. Journal of Nuclear Medicine 53:378–384.
Joshi, A. D., Pontecorvo, M. J., Lu, M., Skovronsky, D. M., Mintun, M. A., & Devous, M. D. (2015). A Semiautomated Method for Quantification of F 18 Florbetapir PET Images. Journal of Nuclear Medicine, 56, 1736–1741.
Klunk, W. E., Koeppe, R. A., Price, J. C., Benzinger, T. L., Devous, M. D., Jagust, W. J., Johnson, K. A., Mathis, C. A., Minhas, D., Pontecorvo, M. J., Rowe, C. C., Skovronsky, D. M., & Mintun, M. A. (2015). The Centiloid Project: standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement, 11, 1-15-4.
Klyuzhin, I. S., Blinder, S., Mabrouk, R., Rahmim, A., & Sossi, V. (2015): Investigation of texture quantification parameters for neurological PET image analysis. IEEE Nuclear Science Symposium and Medical Imaging Conference.
Lambin, P., Rios-Velazquez, E., Leijenaar, R., Carvalho, S., van Stiphout, R. G. P. M., Granton, P., Zegers, C. M. L., Gillies, R., Boellard, R., Dekker, A., & Aerts, H. J. W. L. (2012). Radiomics: extracting more information from medical images using advanced feature analysis. European Journal of Cancer, 48, 441–446.
Landau, S. M., Fero, A., Baker, S. L., Koeppe, R., Mintun, M., Chen, K., Reiman, E. M., & Jagust, W. J. (2015). Measurement of longitudinal β-amyloid change with 18F-florbetapir PET and standardized uptake value ratios. Journal of Nuclear Medicine, 56, 567–574.
Landau, S. M., Mintun, M. A., Joshi, A. D., Koeppe, R. A., Petersen, R. C., Aisen, P. S., Weiner, M. W., & Jagust, W. J. Alzheimer’s Disease Neuroimaging Initiative (2012): Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Annals of Neurology 72:578–586.
McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., & Stadlan, E. M. (1984). Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology, 34, 939–944.
Minoshima, S., Drzezga, A. E., Barthel, H., Bohnen, N., Djekidel, M., Lewis, D. H., Mathis, C. A., McConathy, J., Nordberg, A., Sabri, O., Seibyl, J. P., Stokes, M. K., & Van Laere, K. (2016). SNMMI Procedure Standard/EANM Practice Guideline for Amyloid PET Imaging of the Brain 1.0. Journal of Nuclear Medicine, 57, 1316–1322.
Miwa, K., Inubushi, M., Wagatsuma, K., Nagao, M., Murata, T., Koyama, M., Koizumi, M., & Sasaki, M. (2014). FDG uptake heterogeneity evaluated by fractal analysis improves the differential diagnosis of pulmonary nodules. European Journal of Radiology, 83, 715–719.
Nemmi, F., Saint-Aubert, L., Adel, D., Salabert, A.-S., Pariente, J., Barbeau, E. J., Payoux, P., & Péran, P. (2014). Insight on AV-45 binding in white and grey matter from histogram analysis: a study on early Alzheimer’s disease patients and healthy subjects. European Journal of Nuclear Medicine and Molecular Imaging, 41, 1408–1418.
Ong, K. T., Villemagne, V. L., Bahar-Fuchs, A., Lamb, F., Langdon, N., Catafau, A. M., Stephens, A. W., Seibyl, J., Dinkelborg, L. M., Reininger, C. B., Putz, B., Rohde, B., Masters, C. L., & Rowe, C. C. (2015). Aβ imaging with 18F-florbetaben in prodromal Alzheimer’s disease: a prospective outcome study. Journal of Neurology, Neurosurgery, and Psychiatry, 86, 431–436.
Orlhac, F., Thézé, B., Soussan, M., Boisgard, R., & Buvat, I. (2016). Multiscale Texture Analysis: From 18F-FDG PET Images to Histologic Images. Journal of Nuclear Medicine, 57, 1823–1828.
Padilla, P., López, M., Górriz, J. M., Ramírez, J., Salas-González, D., & Álvarez, I. Alzheimer’s Disease Neuroimaging Initiative (2012): NMF-SVM based CAD tool applied to functional brain images for the diagnosis of Alzheimer’s disease. IEEE Transactions on Medical Imaging 31:207–216.
Petersen, R. C., Aisen, P., Boeve, B. F., Geda, Y. E., Ivnik, R. J., Knopman, D. S., Mielke, M., Pankratz, V. S., Roberts, R., Rocca, W. A., Weigand, S., Weiner, M., Wiste, H., & Jack, C. R. (2013). Mild cognitive impairment due to Alzheimer disease in the community. Ann Neurol, 74, 199–208.
Petersen, R. C., Aisen, P. S., Beckett, L. A., Donohue, M. C., Gamst, A. C., Harvey, D. J., Jack, C. R., Jagust, W. J., Shaw, L. M., Toga, A. W., Trojanowski, J. Q., & Weiner, M. W. (2010). Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology, 74, 201–209.
Pontecorvo, M. J., & Mintun, M. A. (2011). PET amyloid imaging as a tool for early diagnosis and identifying patients at risk for progression to Alzheimer’s disease. Alzheimer’s Research & Therapy, 3, 11.
Rullmann, M., Dukart, J., Hoffmann, K.-T., Luthardt, J., Tiepolt, S., Patt, M., Gertz, H.-J., Schroeter, M. L., Seibyl, J., Schulz-Schaeffer, W. J., Sabri, O., & Barthel, H. (2016). Partial-Volume Effect Correction Improves Quantitative Analysis of 18F-Florbetaben β-Amyloid PET Scans. Journal of Nuclear Medicine, 57, 198–203.
Saint-Aubert, L., Nemmi, F., Péran, P., Barbeau, E. J., Payoux, P., Chollet, F., & Pariente, J. (2014). Comparison between PET template-based method and MRI-based method for cortical quantification of florbetapir (AV-45) uptake in vivo. European Journal of Nuclear Medicine and Molecular Imaging, 41, 836–843.
Schreiber, S., Landau, S. M., Fero, A., Schreiber, F., & Jagust, W. J. Alzheimer’s Disease Neuroimaging Initiative (2015): Comparison of Visual and Quantitative Florbetapir F 18 Positron Emission Tomography Analysis in Predicting Mild Cognitive Impairment Outcomes. JAMA Neurology 72:1183–1190.
Schwarz, C. G., Senjem, M. L., Gunter, J. L., Tosakulwong, N., Weigand, S. D., Kemp, B. J., Spychalla, A. J., Vemuri, P., Petersen, R. C., Lowe, V. J., & Jack, C. R. (2017). Optimizing PiB-PET SUVR change-over-time measurement by a large-scale analysis of longitudinal reliability, plausibility, separability, and correlation with MMSE. Neuroimage, 144, 113–127.
Shokouhi, S., Mckay, J. W., Baker, S. L., Kang, H., Brill, A. B., Gwirtsman, H. E., Riddle, W. R., Claassen, D. O., & Rogers, B. P. Alzheimer’s Disease Neuroimaging Initiative (2016): Reference tissue normalization in longitudinal (18)F-florbetapir positron emission tomography of late mild cognitive impairment. Alzheimer’s Research & Therapy 8:2.
Shokouhi, S., Rogers, B. P., Kang, H., Ding, Z., Claassen, D. O., Mckay, J. W., & Riddle, W. R. Alzheimer’s Disease Neuroimaging Initiative (2015): Modeling clustered activity increase in amyloid-beta positron emission tomographic images with statistical descriptors. Clinical Interventions in Aging 10:759–770.
Smitha, K. A., Gupta, A. K., & Jayasree, R. S. (2015). Fractal analysis: fractal dimension and lacunarity from MR images for differentiating the grades of glioma. Physics in Medicine and Biology, 60, 6937–6947.
Vapnik, V. N. (1999). An overview of statistical learning theory. IEEE Transactions on Neural Networks, 10, 988–999.
Westman, E., Muehlboeck, J.-S., & Simmons, A. (2012). Combining MRI and CSF measures for classification of Alzheimer’s disease and prediction of mild cognitive impairment conversion. Neuroimage, 62, 229–238.
Acknowledgements
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (http://www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Data used in preparation of this paper were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (http://adni.loni.usc.edu/). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this paper. A complete listing of ADNI investigators can be found at http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
the authors have no conflict of interest to disclose.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ben Bouallègue, F., Vauchot, F., Mariano-Goulart, D. et al. Diagnostic and prognostic value of amyloid PET textural and shape features: comparison with classical semi-quantitative rating in 760 patients from the ADNI-2 database. Brain Imaging and Behavior 13, 111–125 (2019). https://doi.org/10.1007/s11682-018-9833-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-018-9833-0