Abstract
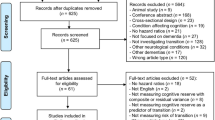
We explored the cross-sectional relationships between β-amyloid (Aβ) and inferior temporal tau deposition (IFT Tau) on cognitive performance and whether cognitive reserve (CR) modifies these associations. We studied 156 participants classified into groups of clinically normal (CN = 133), mild cognitive impairment (MCI = 17) and Alzheimer disease (AD = 6) dementia. AMNART IQ served as a proxy of CR and cognitive performance was assessed using the MMSE. In separate linear regression models predicting MMSE, we examined the interactions of CR x global Aβ and CR x IFT tau across all participants and within the CN group alone. In the whole sample, the interaction between CR and IFT tau was significant (p < 0.003), such that higher CR participants with elevated IFT tau had better MMSE scores compared with low CR participants with similar levels of IFT tau. The interaction between CR and Aβ status did not reach significance (p = 0.093). In CN only, no cross-sectional interactions among CR, Aβ, and IFT tau were observed on MMSE. These findings imply that CR may be protective against early AD processes and enable some individuals to remain cognitively stable despite elevated tau and Aβ burden.


Similar content being viewed by others
References
Arriagada, P. V., Growdon, J. H., Hedley-White, E. T., & Hyman, B. T. (1992a). Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology, 42, 631–639.
Arriagada, P. V., Marzloff, K., & Hyman, B. T. (1992b). Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer's disease. Neurology, 42(9), 1681–1688.
Bateman, R. J., Xiong, C., Benzinger, T. L., Fagan, A. M., Goate, A., Fox, N. C., et al. (2012). Clinical and biomarker changes in dominantly inherited Alzheimer's disease. The New England Journal of Medicine, 367(9), 795–804.
Bennett, D. A., Wilson, R. S., Schneider, J. A., Evans, D. A., Mendes de Leon, C. F., Arnold, S. E., et al. (2003). Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology, 60(12), 1909–1915.
Bennett, D. A., Wilson, R. S., Boyle, P. A., Buchman, A. S., & Schneider, J. A. (2012). Relation of neuropathology to cognition in persons without cognitive impairment. Annals of Neurology, 72(4), 599–609.
Bieliauskas, L. A., Back-Madruga, C., Lindsay, K. L., Wright, E. C., Kronfol, Z., Lok, A. S., et al. (2007). Cognitive reserve and neuropsychological functioning in patients infected with hepatitis C. Journal of the International Neuropsychological Society, 13(4), 687–692.
Braak, H., Alafuzoff, I., Arzberger, T., Kretzschmar, H., & Del Tredici, K. (2006). Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathologica, 112(4), 389–404.
Chien, D. T., Bahri, S., Szardenings, A. K., Walsh, J. C., Mu, F., Su, M. Y., et al. (2013). Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. Journal of Alzheimer's Disease, 34(2), 457–468.
Delacourte, A., Sergeant, N., Wattez, A., Maurage, C. A., Lebert, F., Pasquier, F., et al. (2002). Tau aggregation in the hippocampal formation: an ageing or a pathological process? Experimental Gerontology, 37(10–11), 1291–1296.
Desikan, R. S., Segonne, F., Fischl, B., Quinn, B. T., Dickerson, B. C., Blacker, D., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31(3), 968–980.
Fischl, B., Salat, D. H., Busa, E., Albert, M., Dieterich, M., Haselgrove, C., et al. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355.
Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198.
Gomperts, S. N., Rentz, D. M., Moran, E., Becker, J. A., Locascio, J. J., Klunk, W. E., et al. (2008). Imaging amyloid deposition in Lewy body diseases. Neurology, 71(12), 903–910.
Hardy, J. (2009). The amyloid hypothesis for Alzheimer's disease: a critical reappraisal. Journal of Neurochemistry, 110(4), 1129–1134.
Hedden, T., Oh, H., Younger, A. P., & Patel, T. A. (2013). Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology, 80(14), 1341–1348.
Jack Jr., C. R., Knopman, D. S., Jagust, W. J., Petersen, R. C., Weiner, M. W., Aisen, P. S., et al. (2013). Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurology, 12(2), 207–216.
Johnson, K. A., Schultz, A., Betensky, R. A., Becker, J. A., Sepulcre, J., Rentz, D., et al. (2016). Tau positron emission tomographic imaging in aging and early Alzheimer disease. Annals of Neurology, 79(1), 110–119.
Katzman, R. (1993). Education and the prevalence of dementia and Alzheimer's disease. Neurology, 43, 13–20.
Kemppainen, N. M., Aalto, S., Karrasch, M., Nagren, K., Savisto, N., Oikonen, V., et al. (2008). Cognitive reserve hypothesis: Pittsburgh compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer's disease. Annals of Neurology, 63(1), 112–118.
Klunk, W. E., Engler, H., Nordberg, A., Wang, Y., Blomqvist, G., Holt, D. P., et al. (2004). Imaging brain amyloid in Alzheimer's disease with Pittsburgh compound-B. Annals of Neurology, 55(3), 306–319.
Marquie, M., Normandin, M. D., Vanderburg, C. R., Costantino, I. M., Bien, E. A., Rycyna, L. G., et al. (2015). Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Annals of Neurology, 78(5), 787–800.
McKee, A. C., Cantu, R. C., Nowinski, C. J., Hedley-Whyte, E. T., Gavett, B. E., Budson, A. E., et al. (2009). Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. Journal of Neuropathology and Experimental Neurology, 68(7), 709–735.
Morris, J. C. (1993). The clinical dementia rating scale (CDR): current version and scoring rules. Neurology, 43, 2412–2414.
Mungas, D., Marshall, S. C., Weldon, M., Haan, M., & Reed, B. R. (1996). Age and education correction of mini-mental state examination for English and Spanish-speaking elderly. Neurology, 46(3), 700–706.
Nelson, P. T., Alafuzoff, I., Bigio, E. H., Bouras, C., Braak, H., Cairns, N. J., et al. (2012). Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. Journal of Neuropathology and Experimental Neurology, 71(5), 362–381.
Price, J. L., & Morris, J. C. (1999). Tangles and plaques in nondemented aging and "preclinical" Alzheimer's disease. Annals of Neurology, 45(3), 358–368.
Rentz, D.M., Huh, T.J., Sardinha, L.M., Moran, E.K., Becker, J.A., Daffner, K.A., et al. (2007). Intelligence quotient-adjusted memory impairment is associated with abnormal single photon emission computed tomography perfusion. Journal International Neuropsychological Society 13(5): 821–31.
Rentz, D. M., Locascio, J. J., Becker, J. A., Moran, E. K., Eng, E., Buckner, R. L., et al. (2010). Cognition, reserve, and amyloid deposition in normal aging. Annals of Neurology, 67(3), 353–364.
Roe, C. M., Mintun, M. A., D'Angelo, G., Xiong, C., Grant, E. A., & Morris, J. C. (2008). Alzheimer disease and cognitive reserve: variation of education effect with carbon 11-labeled Pittsburgh compound B uptake. Archives of Neurology, 65(11), 1467–1471.
Rowe, C. C., Ellis, K. A., Rimajova, M., Bourgeat, P., Pike, K. E., Jones, G., et al. (2010). Amyloid imaging results from the Australian imaging, biomarkers and lifestyle (AIBL) study of aging. Neurobiology of Aging, 31(8), 1275–1283.
Ryan, J., & Paolo, A. (1992). A screening procedure for estimating premorbid intelligence in the elderly. The Clinical Neuropsychologist, 6, 53–62.
Scarmeas, N., Zarahn, E., Anderson, K. E., Habeck, C. G., Hilton, J., Flynn, J., et al. (2003). Association of life activities with cerebral blood flow in Alzheimer disease: implications for the cognitive reserve hypothesis. Archives of Neurology, 60(3), 359–365.
Scarmeas, N., Luchsinger, J. A., Schupf, N., Brickman, A. M., Cosentino, S., Tang, M. X., et al. (2009). Physical activity, diet, and risk of Alzheimer disease. JAMA, 302(6), 627–637.
Sperling, R. A., Aisen, P. S., Beckett, L. A., Bennett, D. A., Craft, S., Fagan, A. M., et al. (2011a). Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement, 7(3), 280–292.
Sperling, R. A., Jack Jr., C. R., & Aisen, P. S. (2011b). Testing the right target and right drug at the right stage. Science Translational Medicine, 3(111), 111 cm133.
Stern, Y. (2009). Cognitive reserve. Neuropsychologia, 47(10), 2015–2028.
Stern, Y. (2012). Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurology, 11(11), 1006–1012.
Stern, Y., Alexander, G. E., Prohovnik, I., & Mayeux, R. (1992). Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer's disease. Annals of Neurology, 32, 371–375.
Stern, Y., Alexander, G. E., Prohovnik, I., Stricks, L., Link, B., Lennon, M. C., et al. (1995). Relationship between lifetime occupation and parietal flow: implications for a reserve against Alzheimer's disease pathology. Neurology, 45, 55–60.
Sumowski, J. F., Chiaravalloti, N., & DeLuca, J. (2009). Cognitive reserve protects against cognitive dysfunction in multiple sclerosis. Journal of Clinical and Experimental Neuropsychology, 31(8), 913–926.
Wilson, R. S., Bennett, D. A., Bienias, J. L., Mendes de Leon, C. F., Morris, M. C., & Evans, D. A. (2003a). Cognitive activity and cognitive decline in a biracial community population. Neurology, 61(6), 812–816.
Wilson, R., Barnes, L., & Bennett, D. (2003b). Assessment of lifetime participation in cognitively stimulating activities. Journal of Clinical and Experimental Neuropsychology, 25(5), 634–642.
Wilson, R. S., Scherr, P. A., Schneider, J. A., Tang, Y., & Bennett, D. A. (2007). Relation of cognitive activity to risk of developing Alzheimer disease. Neurology, 69(20), 1911–1920.
Wirth, M., Villeneuve, S., La Joie, R., Marks, S. M., & Jagust, W. J. (2014). Gene–environment interactions: lifetime cognitive activity, APOE genotype, and beta-amyloid burden. The Journal of Neuroscience, 34(25), 8612–8617.
Wolk, D. A., Price, J. C., Madeira, C., Saxton, J. A., Snitz, B. E., Lopez, O. L., et al. (2012). Amyloid imaging in dementias with atypical presentation. Alzheimers Dement, 8(5), 389–398.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding
The National Institute on Aging (P01 AG036694, RO1 AG037497, RO1EB014894) and the Alzheimer’s Association IIRG-08-90,934 funded this study.
Conflict of interest
D. Rentz received research support from the NIH grants P01 AG036694, R01 MH090291, U01 AG024904, R01 AG027435, R01 AG037497 and P50 AG005134, Alzheimer Association grant IIRG-08-90,934 and Fidelity Biosciences. She has also served as a paid consultant for Eli Lilly, Lundbeck Pharmaceuticals, Janssen Alzheimer Immunotherapy, Biogen, Idec and Neurotrack. These relationships are not related to the content in the manuscript.
E. Mormino received funding from NIH grant F32AG044054 and P01 AG036694 and has served as a paid consultant for Janssen Pharmaceuticals and Biogen Idec. These relationships are not related to the content in the manuscript.
K. Papp was supported by NIA grant T32AG023480–08, the Charles King Trust, and NIH grant P01 AG036694 and has served as a paid consultant for Biogen Idec. These relationships are not related to the content in the manuscript.
R. Betensky received funding from NIH grants R01 CA075971, R03 CA165070, UL 1RR025758, P50 NS051343, P50 NS051343, P30 CA006516, P50 AG005134, P01 AG036694, R01 NS070834, R01 NS070834 and R01 AG026484.
R. Sperling has served as a paid consultant for Abbvie, Biogen, Bracket, Genentech, Lundbeck, Roche, and Sanofi. She has served as a co-investigator for Avid, Eli Lilly, and Janssen Alzheimer Immunotherapy clinical trials. She has spoken at symposia sponsored by Eli Lilly, Biogen, and Janssen. R. Sperling receives research support from Janssen Pharmaceuticals, and Eli Lilly and Co. These relationships are not related to the content in the manuscript. She also receives research support from the following grants: P01 AG036694, U01 AG032438, U01 AG024904, R01 AG037497, R01 AG034556, K24 AG035007, P50 AG005134, U19 AG010483, R01 AG027435, Fidelity Biosciences, Harvard NeuroDiscovery Center, and the Alzheimer’s Association.
K. Johnson has served as paid consultant for Bayer, Bristol-Myers Squibb, GE Healthcare, Janssen Alzheimer’s Immunotherapy, Siemens Medical Solutions, and Genzyme. He is a site coinvestigator for Lilly/Avid, Bristol-Myers Squibb, Pfizer, Janssen Immunotherapy, and Navidea. He has spoken at symposia sponsored by Janssen Alzheimer’s Immunotherapy and Pfizer. T. Benzinger has served on an advisory board for Eli Lilly and has received research funding from Avid Radiopharmaceuticals. These relationships are not related to the content in the manuscript. K. Johnson receives funding from NIH grants R01EB014894, R21 AG038994, R01 AG026484, R01 AG034556, P50 AG00513421, U19 AG10483, P01 AG036694, R13 AG042201174210, R01 AG027435, and R01 AG037497 and the Alzheimer’s Association grant ZEN-10-174,210.
Rights and permissions
About this article
Cite this article
Rentz, D.M., Mormino, E.C., Papp, K.V. et al. Cognitive resilience in clinical and preclinical Alzheimer’s disease: the Association of Amyloid and Tau Burden on cognitive performance. Brain Imaging and Behavior 11, 383–390 (2017). https://doi.org/10.1007/s11682-016-9640-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-016-9640-4