Abstract
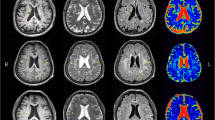
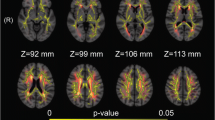
White matter hyperintensities (WMHs) are associated with cognitive decline, but less is known about pathophysiology of cognitive decline in patients with WMHs. We investigated microvasculature and microstructure in WMHs using intravoxel incoherent motion (IVIM) and their associations with cognitive function. Thirty-two subjects with WMHs were enrolled in our study. Fast diffusion coefficient (D*), perfusion fraction (f) and slow diffusion coefficient (D) from IVIM model were compared between regions of WMHs (periventricular WMHs, PWMHs and deep WMHs, DWMHs) and surrounding normal white matter. Multivariate linear model was used to determine the independent factors associated with cognitive function assessed by the Mini Mental State Examination (MMSE) and the standardized coefficient (β) of factors was estimated. D* was significantly lower (4.95 × 10−3 mm2/s versus 8.36 × 10−3 mm2/s in PWMHs and 5.04 × 10−3 mm2/s versus 8.67 × 10−3 mm2/s in DWMHs, both P < 0.001), and f (14.64 % versus 12.01 % in PWMHs and 14.26 % versus 11.31 % in DWMHs, both P < 0.001) and D (1.02 × 10−3 mm2/s versus 0.73 × 10−3 mm2/s in PWMHs and 0.86 × 10−3 mm2/s versus 0.70 × 10−3 mm2/s in DWMHs, both P < 0.001) were significantly higher in WMHs. Only f in PWMHs was independently associated with MMSE (β = 0.443, P = 0.016). The decreased D* and increased D in WMHs were similar to previous findings. The increased f in PWMHs relating with better cognition provides the pathophysiological basis in understanding cognitive decline in patients with WMHs.




Similar content being viewed by others
References
Alosco, M. L., Brickman, A. M., Spitznagel, M. B., Garcia, S. L., Narkhede, A., Griffith, E. Y., et al. (2013). Cerebral perfusion is associated with white matter hyperintensities in older adults with heart failure. Congestive Heart Failure, 19(4), E29–E34.
Au, R., Massaro, J. M., Wolf, P. A., Young, M. E., Beiser, A., Seshadri, S., et al. (2006). Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart Study. Archives of Neurology, 63(2), 246–250.
Brickman, A. M., Zahra, A., Muraskin, J., Steffener, J., Holland, C. M., Habeck, C., et al. (2009). Reduction in cerebral blood flow in areas appearing as white matter hyperintensities on magnetic resonance imaging. Psychiatry Research, 172(2), 117–120.
Brown, W. R., & Thore, C. R. (2011). Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathology and Applied Neurobiology, 37(1), 56–74.
Covarrubias, D. J., Rosen, B. R., & Lev, M. H. (2004). Dynamic magnetic resonance perfusion imaging of brain tumors. The Oncologist, 9(5), 528–537.
De Groot, J. C., De Leeuw, F. E., Oudkerk, M., Van Gijn, J., Hofman, A., Jolles, J., et al. (2002). Periventricular cerebral white matter lesions predict rate of cognitive decline. Annals of Neurology, 52(3), 335–341.
De Leeuw, F.-E., De Groot, J., Bots, M., Witteman, J., Oudkerk, M., Hofman, A., et al. (2000). Carotid atherosclerosis and cerebral white matter lesions in a population based magnetic resonance imaging study. Journal of Neurology, 247(4), 291–296.
Debette, S., & Markus, H. (2010). The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ, 341, c3666.
Dufouil, C., Godin, O., Chalmers, J., Coskun, O., MacMahon, S., Tzourio-Mazoyer, N., et al. (2009). Severe cerebral white matter hyperintensities predict severe cognitive decline in patients with cerebrovascular disease history. Stroke, 40(6), 2219–2221.
Fazekas, F., Chawluk, J. B., Alavi, A., Hurtig, H., & Zimmerman, R. (1987). MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJNR. American Journal of Neuroradiology, 149(2), 351–356.
Fazekas, F., Kleinert, R., Offenbacher, H., Schmidt, R., Kleinert, G., Payer, F., et al. (1993). Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology, 43(9), 1683–1689.
Federau, C., Maeder, P., O’Brien, K., Browaeys, P., Meuli, R., & Hagmann, P. (2012). Quantitative measurement of brain perfusion with intravoxel incoherent motion MR imaging. Radiology, 265(3), 874–881.
Federau, C., O’Brien, K., Meuli, R., Hagmann, P., & Maeder, P. (2014). Measuring brain perfusion with intravoxel incoherent motion (IVIM): initial clinical experience. Journal of Magnetic Resonance Imaging, 39(3), 624–632.
Filley, C. M. (1998). The behavioral neurology of cerebral white matter. Neurology, 50(6), 1535–1540.
Fritzsche, K. H., Neher, P. F., Reicht, I., van Bruggen, T., Goch, C., Reisert, M., et al. (2012). MITK diffusion imaging. Methods of Information in Medicine, 51(5), 441.
Grueter, B. E., & Schulz, U. G. (2012). Age-related cerebral white matter disease (leukoaraiosis): a review. Postgraduate Medical Journal, 88(1036), 79–87.
Haller, S., Kovari, E., Herrmann, F. R., Cuvinciuc, V., Tomm, A.-M., Zulian, G. B., et al. (2013). Do brain T2/FLAIR white matter hyperintensities correspond to myelin loss in normal aging? A radiologic-neuropathologic correlation study. Acta Neuropathologica Communications, 1(1), 14.
Helenius, J., Soinne, L., Salonen, O., Kaste, M., & Tatlisumak, T. (2002). Leukoaraiosis, ischemic stroke, and normal white matter on diffusion-weighted MRI. Stroke, 33(1), 45–50.
Hu, Y.-C., Yan, L.-F., Wu, L., Du, P., Chen, B.-Y., Wang, L., et al. (2014). Intravoxel incoherent motion diffusion-weighted MR imaging of gliomas: efficacy in preoperative grading. Scientific Reports, 4, 7208.
Kennedy, K. M., & Raz, N. (2009). Pattern of normal age-related regional differences in white matter microstructure is modified by vascular risk. Brain Research, 1297, 41–56.
Le Bihan, D. (2008). Intravoxel incoherent motion perfusion MR imaging: a wake-up call. Radiology, 249(3), 748–752.
Le Bihan, D., & Turner, R. (1992). The capillary network: a link between IVIM and classical perfusion. Magnetic Resonance in Medicine, 27(1), 171–178.
Le Bihan, D., Breton, E., Lallemand, D., Aubin, M., Vignaud, J., & Laval-Jeantet, M. (1988). Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology, 168(2), 497–505.
Maniega, S. M., Hernández, M. C. V., Clayden, J. D., Royle, N. A., Murray, C., Morris, Z., et al. (2015). White matter hyperintensities and normal-appearing white matter integrity in the aging brain. Neurobiology of Aging, 36(2), 909–918.
Markus, H., Lythgoe, D., Ostegaard, L., O’sullivan, M., & Williams, S. (2000). Reduced cerebral blood flow in white matter in ischaemic leukoaraiosis demonstrated using quantitative exogenous contrast based perfusion MRI. Journal of Neurology, Neurosurgery and Psychiatry, 69(1), 48–53.
Marstrand, J., Garde, E., Rostrup, E., Ring, P., Rosenbaum, S., Mortensen, E. L., et al. (2002). Cerebral perfusion and cerebrovascular reactivity are reduced in white matter hyperintensities. Stroke, 33(4), 972–976.
Moody, D. M., Brown, W. R., Challa, V. R., & Anderson, R. L. (1995). Periventricular venous collagenosis: association with leukoaraiosis. Radiology, 194(2), 469–476.
Moody, D. M., Thore, C. R., Anstrom, J. A., Challa, V. R., Langefeld, C. D., & Brown, W. R. (2004). Quantification of afferent vessels shows reduced brain vascular density in subjects with leukoaraiosis. Radiology, 233(3), 883–890.
O’Sullivan, M., Jones, D. K., Summers, P., Morris, R., Williams, S., & Markus, H. (2001). Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology, 57(4), 632–638.
Pantoni, L. (2010). Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurology, 9(7), 689–701.
Powers, W. J., Grubb, R. L., & Raichle, M. E. (1984). Physiological responses to focal cerebral ischemia in humans. Annals of Neurology, 16(5), 546–552.
Prins, N. D., & Scheltens, P. (2015). White matter hyperintensities, cognitive impairment and dementia: an update. Nature Reviews Neurology, 11(3), 157–165.
Sabri, O., Ringelstein, E.-B., Hellwig, D., Schneider, R., Schreckenberger, M., Kaiser, H.-J., et al. (1999). Neuropsychological impairment correlates with hypoperfusion and hypometabolism but not with severity of white matter lesions on MRI in patients with cerebral microangiopathy. Stroke, 30(3), 556–566.
Schmidt, P., Gaser, C., Arsic, M., Buck, D., Förschler, A., Berthele, A., et al. (2012). An automated tool for detection of FLAIR-hyperintense white-matter lesions in multiple sclerosis. NeuroImage, 59(4), 3774–3783.
Taheri, S., Gasparovic, C., Huisa, B. N., Adair, J. C., Edmonds, E., Prestopnik, J., et al. (2011). Blood–brain barrier permeability abnormalities in vascular cognitive impairment. Stroke, 42(8), 2158–2163.
Taylor, W. D., Payne, M. E., Krishnan, K. R. R., Wagner, H. R., Provenzale, J. M., Steffens, D. C., et al. (2001). Evidence of white matter tract disruption in MRI hyperintensities. Biological Psychiatry, 50(3), 179–183.
Tuladhar, A. M., Reid, A. T., Shumskaya, E., de Laat, K. F., van Norden, A. G., van Dijk, E. J., et al. (2015). Relationship between white matter hyperintensities, cortical thickness, and cognition. Stroke, 46(2), 425–432.
Wardlaw, J. M., Smith, E. E., Biessels, G. J., Cordonnier, C., Fazekas, F., Frayne, R., et al. (2013). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurology, 12(8), 822–838.
Wirestam, R., Borg, M., Brockstedt, S., Lindgren, A., Holtås, S., & Ståhlberg, F. (2001). Perfusion-related parameters in intravoxel incoherent motion MR imaging compared with CBV and CBF measured by dynamic susceptibility-contrast MR technique. Acta Radiologica, 42(2), 123–128.
Wu, W.-C., Chen, Y.-F., Tseng, H.-M., & Yang, S.-C. (2015). Caveat of measuring perfusion indexes using intravoxel incoherent motion magnetic resonance imaging in the human brain. European Radiology, 25(8), 2485–2492.
Wurnig, M. C., Donati, O. F., Ulbrich, E., Filli, L., Kenkel, D., Thoeny, H. C., et al. (2015). Systematic analysis of the intravoxel incoherent motion threshold separating perfusion and diffusion effects: proposal of a standardized algorithm. Magnetic Resonance in Medicine, 74(5), 1414–1422.
Yamada, K., Sakai, K., Owada, K., Mineura, K., & Nishimura, T. (2010). Cerebral white matter lesions may be partially reversible in patients with carotid artery stenosis. AJNR. American Journal of Neuroradiology, 31(7), 1350–1352.
Yamaji, S., Ishii, K., Sasaki, M., Imamura, T., Kitagaki, H., Sakamoto, S., et al. (1997). Changes in cerebral blood flow and oxygen metabolism related to magnetic resonance imaging white matter hyperintensities in Alzheimer’s disease. Journal of Nuclear Medicine, 38(9), 1471–1474.
Yan, S., Wan, J., Zhang, X., Tong, L., Zhao, S., Sun, J., et al. (2014). Increased visibility of deep medullary veins in leukoaraiosis: a 3-T MRI study. Frontiers in Aging Neuroscience, 6, 144.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Zhejiang Provincial Natural Science Foundation of China (grant number: LZ14H180001), National Natural Science Foundation of China (grant number: 81271530) and Health and Family Planning Commission of Zhejiang Province (grant number: 2016154942).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
“All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.”
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 229 kb)
Rights and permissions
About this article
Cite this article
Sun, J., Yu, X., Jiaerken, Y. et al. The relationship between microvasculature in white matter hyperintensities and cognitive function. Brain Imaging and Behavior 11, 503–511 (2017). https://doi.org/10.1007/s11682-016-9531-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-016-9531-8