Abstract
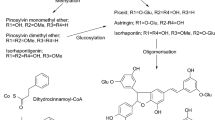
Macroscopic fungi on Caucasian alder wood (Alnus subcordata) were identified and tested as a source of betulin and betulinic acid (the most important metabolites of the Betulaceae family) to evaluate levels of phenols, flavonoids and antioxidant activity. Ganoderma applanatum, Lenzites betulina, Trichaptum biforme, Rigidoporus ulamrius, Fomes fomentarius, Schizophyllum commune, Auricularia mesenterica, and Trametes versicolor were among those identified, and they differed significantly in the level of betulin and betulinic acid and phenols, flavonoids and antioxidant properties in fungal tissues extracted with methanol and with ethanol (p ≤ 0.01). G. applanatum had the most betulin (3.642%) and S. commune the most betulinic acid (1.413%). All tested fungi had high antioxidant activity, and L. betulina had the highest (97.775%). The highest amounts of phenol (719.993 mg mL−1) and flavonoids (361.403 mg mL−1) were found in the ethanolic extract from G. applanatum. Considering the results of this study and the low cost and convenient access to these fungi, they should be good sources for producing different drugs.











Similar content being viewed by others
Change history
21 July 2020
As determined through 11 subcultures of Taxus cuspidata callus, the growth in FW and level of Taxol production were stable by the ninth generation.
References
Ajith TA, Janardhanan KK (2007) Indian medicinal mushroom as a source of antioxidant and antitumor agents. J Clin Biochem Nutr 40(3):157–162
Bai YH, Feng YQ, Mao DB, Xu CP (2012) Optimization for betulin production from mycelia culture of Inonotus obliquus by orthogonal design and evaluation of its antioxidant activity. J Taiwan Inst Chem Eng 43:663–669
Barbieri A, Quagliariello V, Vecchio VD, Falco M, Luciano A, Amruthraj NJ, Nasti G, Ottaiano A, Berretta M, Iaffaioli RV, Arra C (2017) Anticancer and anti-inflammatory properties of Ganoderma lucidum extract effects on melanoma and triple-negative breast cancer treatment. Nutrients 9(3):pii: E210. https://doi.org/10.3390/nu9030210
Cichewicz RH, Kouzi SA (2004) Chemistry, biological activity, and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection. Med Care Res Rev 24:90–114
Daniel A, McElhenney WH (2013) Extraction of total phenolic and flavonoids from edible wild and cultivated medicinal mushrooms as affected by different solvents. J Nat Prod Plant Resour 3(3):37–42
Faass N (2012) The healing powers of wild chaga; an interview with Cass Ingram. MD. Price-Pottenger. J Health Heal 35(4):6–11
Feng Y, Li M, Liu J, Xu TY, Fang RS, Chen QH, He GQ (2013) A novel one-step microbial transformation of betulin to betulinic acid catalysed by Cunningham Ella blakesleeana. Food Chem 136:73–79
Jasicka-Misiak I, Lipok J, Swider I, Kafarski P (2010) Possible fungistatic implications of betulin presence in Betulaceae plants and their Hymenochaetaceae parasitic fungi. Z Naturforsch C 65(3–4):201–206
Joel EL, Bhimba BV (2013) A secondary metabolite with antibacterial activity produced by mangrove foliar fungus Schizophyllum commune. Int J Chem Environ Biol Sci 1(1):165–168
Jonathan SG, Fasidi O (2003) Antimicrobial activities of two Nigerian edible macro-fungi Lycoperdon pusilum and lycoperdon giganteum Pers. Afr J Biomed Res 6:85–90
Kabanov AS, Kosogova TA, Shishkina LN, Tepliakova TV, Skarnovich MO, Mazurkova NA, Puchkova LI, Malkova EM, Stavskii EA, Drozdov IG (2011) Study of antiviral activity of extracts obtained from basidial fungi against influenza viruses of different subtypes in experiments in vitro and in vivo. Zh Mikrobiol Epidemiol Immunobiol 1:40–43
Kahlos K (1994) Inonotus obliquus (Chaga Fungus): in vitro culture and the production of Inotodiol, Sterols, and other secondary metabolites. J For 26:179–198
Kao CHJ, Jesuthasan AC, Bishop KS, Glucina MP, Ferguson LP (2013) Anti-cancer activities of Ganoderma lucidum: active ingredients and pathways. Funct Foods Health Dis 3:48–65
Kessler JH, Mullauer FB, Roo GM, Medema JP (2007) Broad in vitro efficacy of plant-derived betulinic acid against cell lines derived from the most prevalent human cancer types. Cancer Lett 251:132–145
Krasutsky PA (2006) Birch bark research and development. Nat Prod Res 23:919–942
Lin ZB (2005) Cellular and molecular mechanisms of immuno-modulation by Ganoderma lucidum. J Pharmacol Sci 99:144–153
Manasseh AT, Godwin JT, Emanghe AEU, Borisde OO (2012) Phytochemical properties of Ganoderma applanatum as potential agents in the application of nanotechnology in modern day medical practice. Asian Pac J Trop Biomed 2(2):580–583
Mashayekhi K, Atashi S (2014) The analyzing methods in plant physiology. Sirang Press, Gorgan, p 310
Nazari J, Payamnoor V, Kavosi MR (2016) The evaluation of the absorption of some secondary metabolites (betulin, betulinic acid, phenol, flavonoids) and antioxidant activity of wood-inhabiting fungi on Betula pendula (L) Roth. in Golestan province. Eco Phytochem J Med Plants 4(2):44–55
Paterson RRM (2006) Ganoderma—a therapeutic fungal biofactory. Phytochemistry 67(18):1985–2001
Rajoriya A, Tripathy SS, Gupta N (2015) In vitro antioxidant activity of selected Ganoderma species found in Odisha. India. J Trop Plant Res 2(2):72–77
Rice-Evans C (2004) Flavonoids and isoflavones (absorption, metabolism and bioactivity). Free Radic Biol Med 36:827–828
Saadatmand S (2007) Modern mycology. Ayandegan Press, Tehran, p 176
Tabari SH, Ghorbanli M, Safaian Sh, Moosazade S (2013) Comparison of antioxidant and phytochemical properties of Trametes gibbosa. J Cell Mol Biotech 10(3):73–78
Teoh YP, Don MM (2013) In vitro antifungal activities and phytochemical analysis of filamentous white-rot fungi, Schizophyllum commune. Sains Malays 42(9):1267–1272
Teplyakova TV, Psurtseva NV, Kosogova TA, Mazurkova NA, Khanin VA, Vlasenko VA (2012) Antiviral activity of polyporoid mushrooms (higher Basidiomycetes) from Altai Mountains (Russia). Int J Med Mushrooms 14(1):37–45
Wu H, Yang HY, You XL, Li YH (2012) Isolation and characterization of saponin-producing fungal endophytes from Aralia elata in Northeast China. Int J Mol Sci 13:16255–16266
Yin YG, Cui YR, Ding HW (2007) Optimization of betulin extraction process from Inonotus Obliquus with pulsed electric fields. Innov Food Sci Emerg Technol 9:306–310
Zhao GL, Yan WD, Cao D (2007) Simultaneous determination of Betulin and Betulinic acid in hite birch bark using RP–HPLC. J Pharm Biomed Anal 43:959–962
Author information
Authors and Affiliations
Corresponding author
Additional information
Corresponding editor: Tao Xu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: The work was supported by Gorgan university of agricultural sciences and natural resources - grant no. 93-323-28.
The online version is available at http://www.springerlink.com
Rights and permissions
About this article
Cite this article
Payamnoor, V., Kavosi, M. & Nazari, J. Polypore fungi of Caucasian alder as a source of antioxidant and antitumor agents. J. For. Res. 31, 1381–1390 (2020). https://doi.org/10.1007/s11676-019-00892-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-019-00892-2