Abstract
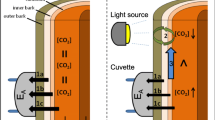
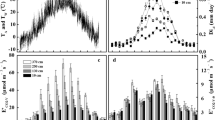
The CO2 released from respiring cells in woody tissues of trees can contribute to one of three fluxes: efflux to the atmosphere (EA), internal xylem sap transport flux (FT), and storage flux (∆S). Adding those fluxes together provides an estimate of actual stem respiration (RS).We know that the relative proportion of CO2 in those fluxes varies greatly among tree species, but we do not yet have a clear understanding of the causes for this variation. One possible explanation is that species differ in stem radial CO2 conductance (gc). A high gc would favor the EA pathway and a low gc would favor the FT pathway. However, gc has only been measured once in situ and only in a single tree species. We measured gc using two methods in stems of Fraxinus mandshurica Rupr. (ash) and Betula platyphylla Suk. (birch) trees in situ, along with RS, EA, FT and ∆S. Stem radial CO2 conductance was substantially greater in ash trees than in birch trees. Corresponding to that finding, in ash trees over 24 h, EA constituted the entire flux of respired CO2, and FT was negative, indicating that additional CO2, probably transported from the root system via the xylem, was also diffusing into the atmosphere. In ash trees, FT was negative over the entire 24 h, and this study represents the first time that has been reported. The addition of xylem-transported CO2 to EA caused EA to be 9% higher than the actual RS over the diel measurement period. Birch trees, which had lower gc, also had a more commonly seen pattern, with EA accounting for about 80% of the CO2 released from local cell respiration and FT accounting for the remainder. The inorganic carbon concentration in xylem sap was also lower in ash trees than in birch trees: 2.7 versus 5.3 mmol L−1, respectively. Our results indicate that stem CO2 conductance could be a very useful measurement to help explain differences among species in the proportion of respired CO2that remains in the xylem or diffuses into the atmosphere.


Similar content being viewed by others
References
Aubrey DP, Teskey RO (2009) Root-derived CO2 efflux via xylem stream rivals soil CO2 efflux. New Phytol 184:35–40
Aubrey DP, Boyles JG, Krysinsky LS, Teskey RO (2011) Spatial and temporal patterns of xylem sap pH derived from stems and twigs of Populus deltoides L. Environ Exp Bot 71:376–381. https://doi.org/10.1016/j.envexpbot.2011.02.006
Butler JN (1991) Carbon dioxide equilibria and their applications. Lewis, Chelsea
Campbell GS, Norman JM (1998) An introduction to environmental biophysics. Springer, New York
Cavaleri MA, Oberbauer SF, Ryan MG (2006) Wood CO2 efflux in a primary tropical rain forest. Glob Change Biol 12(12):2442–2458
Granier A (1985) Une nouvelle méthode pour la mesure du flux de sève brute dans le tronc des arbres. Ann Sci For 42:193–200
Gromping U (2006) Relative importance for linear regression in R: the package relaimpo. J Stat Softw 17:1–27
Hari P, Nygren P, Korpilahti E (1991) Internal circulation of carbon within a tree. Can J For Res 21(4):514–515
Lavigne MB, Ryan MG (1997) Growth and maintenance respiration rates of aspen, black spruce and jack pine stems at northern and southern BOREAS sites. Tree Physiol 17(8–9):543–551
Lendzian KJ (2006) Survival strategies of plants during secondary growth: Barrier properties of phellems and lenticels towards water, oxygen, and carbon dioxide. J Exp Bot 57:2535–2546
McGuire MA, Teskey RO (2002) Microelectrode technique for in situ measurement of carbon dioxide concentrations in xylem sap of trees. Tree Physiol 22:807–811
McGuire MA, Teskey RO (2004) Estimating stem respiration in trees by a mass balance approach that accounts for internal and external fluxes of CO2. Tree Physiol 24:571–578
Panshin AJ, de Zeeuw C (1980) Textbook of wood technology, 4th edn. McGraw-Hill, New York
Salomón RL, Valbuena-Carabaña M, Gil L, McGuire MA, Teskey RO, Aubrey DP, González-Doncel I, Rodríguez-Calcerrada J (2016) Temporal and spatial patterns of internal and external stem CO2 fluxes in a sub-Mediterranean oak. Tree Physiol. https://doi.org/10.1093/treephys/tpw029
Schonherr J, Ziegler H (1980) Water permeability of Betula periderm. Planta 147:345–354
Sorz J, Hietz P (2006) Gas diffusion throughwood: implications for oxygen supply. Trees Struct Funct 20:34–41
Sprugel DG (1990) Components of woody-tissue respiration in young Abies amabilis Forbes trees. Trees Struct Funct 4:88–98
Steppe K, Saveyn A, McGuire MA, Lemeur R, Teskey RO (2007) Resistance to radial CO2 diffusion contributes to between-tree variation in CO2 efflux of Populus deltoides stems. Funct Plant Biol 34:785–792
Teskey RO, McGuire MA (2007) Measurement of stem respiration of sycamore (Platanus occidentalis L.) trees involves internal and external fluxes of CO2 and possible transport of CO2 from roots. Plant Cell Environ 30:570–579
Teskey RO, Saveyn A, Steppe K, McGuire MA (2008) Origin, fate and significance of CO2 in tree stems. New Phytol 177:17–32
Wittmann C, Pfanz H, Loreto F, Centritto M, Pietrini F, Alessio G (2006) Stem CO2 release under illumination: corticular photosynthesis, photorespiration or inhibition of mitochondrial respiration? Plant, Cell Environ 29:1149–1158
Yang JY, Teskey RO, Wang CK (2012a) Stem CO2 efflux of ten species in temperate forests in northeastern China. Trees Struct Funct 26:1225–1235
Yang QP, Xu M, Chi YG, Zheng YP, Shen RC, Li PX, Dai HT (2012b) Temporal and spatial variations of stem CO2 efflux of three species in subtropical China. J Plant Ecol 5:229–237
Zhang ZX (2010) Dendrology—the north, 2nd edn. China Forestry Publishing House, Beijing
Author information
Authors and Affiliations
Corresponding author
Additional information
Project funding: The work was supported by the National Natural Science Foundation of China (31670476 and 31100284) and the Fundamental Research Funds for the Central Universities (2572016CA02).
The online version is available at http://www.springerlink.com
Corresponding editor: Tao Xu.
Rights and permissions
About this article
Cite this article
Wang, X., Mao, Z., McGuire, M.A. et al. Stem radial CO2 conductance affects stem respiratory CO2 fluxes in ash and birch trees. J. For. Res. 30, 21–29 (2019). https://doi.org/10.1007/s11676-018-0737-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-018-0737-z