Abstract
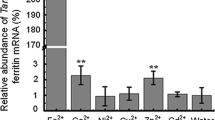
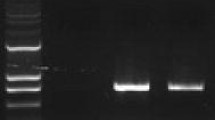
Ferritin, a universal intracellular protein, can store large amounts of iron and improve plant resistance to abiotic and biotic stress. In this study, a ferritin gene (TaFer) from Tamarix androssowii Litv. was transferred into Populus tomentosa Carr. cv ‘BJR01’ via Agrobacterium. Six independent transgenic lines were obtained with a tolerance to kanamycin and three were randomly selected for further analysis. The PCR and RT-PCR results indicate that the TaFer gene had been integrated into the poplar genome. The effect of the gene on abiotic stress tolerance was tested, and the results show that transgenic plants improve growth, had higher chlorophyll and lower MDA contents, and higher relative electrical conductivity, fewer changes of SOD and POD activities, higher iron content, higher root ferric reductase activity and lower levels of ROS accumulation and cell death in response to drought, Fe-insufficient or Fe-excess tolerance. These results indicate that the TaFer gene can improve abiotic stress tolerance in transgenic Populus tomentosa.








Similar content being viewed by others
References
Abadía J, Vázquez S, Rellán-Álvarez R, El-Jendoubi H, Abadía A, Álvarez-Fernández A, López-Millán AF (2011) Towards a knowledge-based correction of iron chlorosis. Plant Physiol Biochem 49:471–482
Agastian P, Kingsley SJ, Vivekanandan M (2000) Effect of salinity on photosynthesis and biochemical characteristics in mulberry genotypes. Photosynthetica 38:287–290
Aisen P, Enns C, Wessling-Resnick M (2001) Chemistry and biology of eukaryotic iron metabolism. Int J Biochem Cell Biol 33:940–959
Andrews SC, Harrison PM, Yewdall SJ, Arosio P, Levi S, Bottke W, von Darl M, Briat JF, Laulhère JP, Lobreaux S (1992) Structure, function, and evolution of ferritins. J Inorg Biochem 47:161–174
Becana M, Moran JF, Iturbe-Ormaetxe I (1998) Iron-dependent oxygen free radical generation in plants subjected to environmental stress: toxicity and antioxidant protection. Plant Soil 201:137–147
Bowler C, Montagu MV, Inzé D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 43:83–116
Briat JF (1996) Roles of ferritin in plants. J Plant Nutr 19:1331–1342
Briat JF (2002) Metal iron mediated oxidative stress and its control. In: Montagu M, Inzé D (eds) Oxidative stress in plants. Taylor and Francis, London, pp 171–189
Briat JF, Fobis-Loisy I, Grignon N, Lobréaux S, Pascal N, Savino G, Thoiron S, von Wirén N, Van Wuytswinkel O (1995) Cellular and molecular aspects of iron metabolism in plants. Biol Cell 84:69–81
Briat JF, Duc C, Ravet K, Gaymard F (2009) Ferritins and iron storage in plants. Biochim Biophys Acta 1800:806–814
Chen Y, Barak P (1982) Iron nutrition of plants in calcareous soils. Adv Agron 135:217–240
Curie C, Briat JF (2003) Iron transport and signaling in plants. Annu Rev Plant Biol 54:183–206
Deák M, Horváth GV, Davletova S, Török K, Sass L, Vass I, Barna B, Király Z, Dudits D (1999) Plants ectopically expressing the iron-binding protein, ferritin, are tolerant to oxidative damage and pathogens. Nat Biotechnol 17(2):192–196
Du NX, Liu X, Li Y, Chen SY, Zhang JS, Ha D, Deng WG, Sun CK, Zhang YZ, Pijut PM (2012) Genetic transformation of Populustomentosa to improve salt tolerance. Plant Cell Tissue Organ Cult 108:181–189
Fridovich I (1995) Superoxide readical and superoxide dismutases. Annu Rev Biochem 64:250–272
Galaris D, Pantopoulos K (2008) Oxidative stress and iron homeostasis: mechanistic and health aspects. Crit Rev Clin Lab Sci 45:1–23
Goto F, Yoshihara T, Saiki H (1998) Iron accumulation in tobacco plants expressing soyabean ferritin gene. Transgenic Res 7:173–180
Goto F, Yoshihara T, Masuda T, Takaiwa F (2001) Genetic improvement of iron content and stress adaptation in plants using ferritin gene. Biotechnol Genet Eng Rev 18:351–372
Guerinot ML (2007) It’s elementary: enhancing Fe3+ reduction improves rice yields. Proc Natl Acad Sci USA 104:7311–7312
Hegedüs A, Janda T, Horváth VG, Dudits D (2008) Accumulation of overproduced ferritin in the chloroplast provides protection against photoinhibition induced by low temperature in tobacco plants. J Plant Physiol 165:1647–1651
Hideg É, Török K, Šnyrychová I, Sándor G, Szegedi E, Horváth VG (2007) Response of ferritin over-expressing tobacco plants to oxidative stress, vol 91. Springer, Berlin, pp 1469–1472
Horsch RB, Hoffmann NL, Eicholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227:1229–1231
Inzé D, Montagu MV (1995) Oxidative Stress in Plants. Curr Opin Biotechnol 6:153–158
Jiang TB, Ding BJ, Li FJ, Yang CP (2006) Differential expression of endogenous ferritin genes and iron homeostasis alteration in transgenic tobacco overexpressing soybean ferritin gene. Acta Genet Sin 33(12):1120–1126
Kangasjärvi S, Neukermans J, Li SC, Aro E-M, Noctor G (2012) Photosynthesis, photorespiration, and light signalling in defence responses. J Exp Bot 63:1619–1636
Kell DB (2009) Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med Genom 2:1–79
Koppenol WH (1993) The centennial of the Fenton reaction. Free Radic Biol Med 15:645–651
Laulhere JP, Briat JF (1993) Iron release and uptake by plant ferritin: effects of pH, reduction and chelation. Biochem J 290:693–696
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Majerus V, Bertin P, Lutts S (2007) Effects of iron toxicity on osmotic potential, osmolytes and polyamines concentrations in the African rice (Oryza glaberrima Steud.). Plant Sci 173(2):96–105
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Papanikolaou G, Pantopoulos K (2005) Iron metabolism and toxicity. Toxicol Appl Pharmacol 202:199–211
Peng XX, Yamauchi M (1993) Ethylene production in rice bronzing leaves induced by ferrous iron. Plant Soil 149:227–234
Peyret P, Perez P, Alric M (1995) Structure, genomic organization, and expression of the Arabidopsis thaliana aconitase gene. Plant aconitase show significant homology with mammalian iron-responsive element-binding protein. J Biol Chem 270:8131–8137
Proudhon D, Briat JF, Lescure AM (1989) Iron induction of ferritin synthesis in soybean cell suspensions. Plant Physiol 90:586–590
Römheld V, Marschner H (1981) Iron deficiency stress induced morphological and physiological changes in root tips of sunflower. Physiol Plant 53:354–360
Römheld V, Marschner H (1986) Evidence for a specific uptake system for iron phytosiderophores in roots of grasses. Plant Physiol 80:175–180
Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW (1984) Ribosomal DNA spacer-length polymorphisms in barley: mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci USA 81:8014–8018
Schmidt W (1999) Mechanisms and regulation of reduction-based iron uptake in plants. New Phytol 141:1–26
Shalata A, Tal M (1998) The effect of salt stress on lipid peroxidation and antioxidants in the leaf of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii. Physiol Plant 104(2):167–174
Sijmons PC, Van den Briel W, Bienfait HF (1984) Cytosolic NADPH is the electron donor for extracellular FeIII reduction in iron-deficient bean roots. Plant Physiol 75:219–221
Sinha S, Gupta M, Chandra P (1997) Oxidative stress induced by iron in Hydrilla verticillata (l.f.) royle: response of antioxidants. Ecotoxicol Environ Saf 138:286–291
Skriver K, Mundy J (1990) Gene expression in response to abscisic acid and osmotic stress. Plant Cell 2:503–512
Spiller S, Kaufman LS, Thomsom WF, Briggs WR (1987) Specific mRNA and rRNA levels in greening pea leaves during recovery from iron stress. Plant Physiol 84:409–414
Terry N, Abadía J (1986) Function of iron in chloroplasts. J Plant Nutr 9:609–646
Theil EC (2003) Ferritin: at the crossroads of iron and oxygen metabolism. J Nutr 133:1549S–1553S
Theil EC (2007) Coordinating responses to iron and oxygen stress with DNA and mRNA promoters: the ferritin story. Biometals 20:513–521
Van Wuytswinkel O, Vansuyt G, Grignon N, Fourcroy P, Briat JF (1998) Iron homeostasis alteration in transgenic tobacco overexpressing ferritin. Plant J 17(1):93–97
Vose PB (1982) Iron nutrition in plants: a word overview. J Plant Nutr 5:233–249
Wang YC, Jiang J, Zhao X, Liu GF, Yang CP, Zhan LP (2006) A novel lea gene from Tamarix androssowii confers drought tolerance in transgenic tobacco. Plant Sci 171:655–662
Yang GY, Wang YC, Xia DA, Gao CQ, Wang C, Yang CP (2014) Overexpression of a GST gene (ThGSTZ1) from Tamarix hispida improves drought and salinity tolerance by enhancing the ability to scavenge reactive oxygen species. Plant Cell Tissue Organ Cult 117:99–112
Yang JL, Chen Z, Wu SQ, CuiY ZL, Dong H, Yang CP, Li CH (2015) Overexpression of the Tamarix hispida ThMT3 gene increases copper tolerance and adventitious root induction in Salix matsudana Koidz. Plant Cell Tissue Organ Cult 121:469–479
Yi Y, Guerinot ML (1996) Genetic evidence that induction of root Fe (III) chelate reductase activity is necessary for iron uptake under iron deficiency. Plant J 10(5):835–844
Zhang TT, Song YZ, Liu YD, Guo XQ, Zhu CX, Wen FJ (2008) Overexpression of phospholipase Dα gene enhances drought and salt tolerance of Populus tomentosa. Chin Sci Bull 53:3656–3665
Zhang X, Wang L, Meng H, Wen HT, Fan YL, Zhao J (2011) Maize ABP9 enhances tolerance to multiple stresses in transgenic Arabidopsis by modulating ABA signaling and cellular levels of reactive oxygen species. Plant Mol Biol 75(4):365–378
Zheng HQ, Lin SZ, Zhang Q, Lei Y, Hou L, Zhang ZY (2010) Functional identification and regulation of the PtDrl02 gene promoter from triploid white poplar. Plant Cell Rep 29:449–460
Zok A, Oláh R, Hideg É, Horváth VG, Kós PB, Majer P, Gy V, Szegedi E (2010) Effect of Medicago sativa ferritin gene on stress tolerance in transgenic grapevine. Plant Cell Tissue Organ Cult 100:339–344
Author information
Authors and Affiliations
Corresponding author
Additional information
Project funding: The work was supported by Hi-Tech Research and Development Program of China (2013AA102701) and Excellent Creative Talents Supporting Program of Heilongjiang University of Chinese Medicine (2012RCQ24).
The online version is available at http://www.springerlink.com
Corresponding editor: Tao Xu.
Rights and permissions
About this article
Cite this article
Zhao, B., Yang, J., Yao, W. et al. Over expression of TaFer gene from Tamarix androssowii improves iron and drought tolerance in transgenic Populus tomentosa. J. For. Res. 30, 171–181 (2019). https://doi.org/10.1007/s11676-018-0625-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-018-0625-6