Abstract
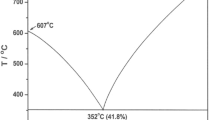
The ternary phase diagram of LiCl-KCl-NdCl3 system has been investigated by differential thermal analysis (DTA), followed by characterization of the coexisting phases in the solid state by x-ray diffraction, in order to understand the interactions in the NdCl3-LiCl-KCl ternary system. The results of these experiments showed that LiCl and K2NdCl5 form a non binary join section. This divides the LiCl-KCl-NdCl3 system into two quasi-ternary sections, namely (1) LiCl-KCl-K2NdCl5 and (2) LiCl-K2NdCl5-NdCl3 systems. Both are simple eutectic ternary phase diagrams. The ternary eutectic temperatures and eutectic compositions are determined to be 316 ± 3 °C and 53.9 mol.% LiCl-38.7 mol.% KCl-7.4 mol.% K2NdCl5 in the LiCl-KCl-K2NdCl5 quasi-ternary section, while the other eutectic temperature and composition are determined to be 376 ± 9 °C and 46.2 mol.% LiCl-32.5 mol.% K2NdCl5-21.3 mol.% NdCl3 in the LiCl-K2NdCl5-NdCl3 quasi-ternary section. A quasi-ternary peritectic reaction is observed at 37.7 mol.% LiCl-36.2 mol.% KCl-26.1 mol.% K2NdCl5 at 445 ± 1°C. The primary and secondary crystallization temperatures for the samples are deduced from the heating runs of DTA traces, and the phases responsible for the various thermal events are ascertained. Isothermal sections at chosen temperatures and polythermal liquidus projection with isothermal contours are drawn over the ternary phase field.

















Similar content being viewed by others
References
J.J. Laidler, J.E. Battles, W.E. Miller, J.P. Ackerman, and E.L. Carls, Development of Pyroprocessing Technology, Prog. Nucl. Energy, 1997, 31, p 131-140
T. Murakami, A. Rodrigues, M. Ougier, M. Iuzika, T. Tsukada, and J.-P. Glatz, Actinides Recovery from Irradiated Metallic Fuel in LiCl-KCl Melts, J. Nucl. Mater., 2015, 466, p 502-508
H. Moriyama, H. Yamana, S. Nishikawa, S. Shibata, N. Wakayama, Y. Miyashita, K. Moritani, and T. Mitsugashora, Thermodynamics of Actinides and Lanthanides from Molten Salt into Liquid Metal, J. Alloy. Compd., 1998, 271–273, p 587-591
T.Y. Gutknecht and G.L. Fredrickson, Thermal characterization of molten salt systems, INL/EXT-11-23511. Idaho National Laboratory, 2011
E.A.C. Crouch, Fission-Product Yields from Neutron Induced Fission, At. Data Nucl. Data, 1977, 19, p 417-532
S. Ghosh, R. Ganesan, R. Sridharan, and T. Gnanasekaran, Study of Phase Equilibria in LiCl-KCl-PrCl3 Ternary System, Thermochim. Acta, 2017, 653, p 16-26
E. Korin and L. Soifer, Thermal Analysis of the System KCl-LiCl by Differential Scanning Calorimetry, J. Therm. Anal., 1997, 50, p 347-354
A.S. Basin, A.B. Kaplun, A.B. Meshalkin, and N.F. Uvarov, The LiCl-KCl Binary System, Russ. J. Inorg. Chem., 2008, 53, p 1509-1511
J. Sangster and A.D. Pelton, Phase Diagrams and Thermodynamic Properties of the 70 Binary Alkali Halide Systems Having Common Ions, J. Phys. Chem. Ref. Data, 1987, 16, p 509-561
S. Ghosh, B.P. Reddy, K. Nagarajan, and K.C. Hari Kumar, Experimental Investigations and Thermodynamic Modelling of KCl–LiCl–UCl3 System, CALPHAD, 2014, 45, p 11-26
A.B. Kaplun and A.B. Meshalkin, Oscillation Method of Phase Analysis: A Precision Method for an Integrated Study of Physicochemical Characteristics and the Process of Crystallisation and Melting, J. Struct. Chem., 2014, 55, p 1172-1179
Y. Zhang, C. Zheng, and Y. Yupu, Phase Diagram of System NdCl3-LiCl-KCl, Acta. Metall. Sin. B, 1989, 2, p 13-17
I-S. Kim and Y. Okamoto, Phase diagrams for Binary Systems NdCl3–LiCl and PrCl3–LiCl, JAERI-Research 99-033, 1999
Y. Sun, X. Ye, Y. Wang, and J. Tan, Optimisation and Calculation of the NdCl3-MCl (M = Li, Na, K, Rb, Cs) Phase Diagrams, CALPHAD, 2004, 28, p 109-114
W. Gong, M. Gaune-Escard, and L. Rycerz, Thermodynamic Assessment of LiCl-NdCl3 and LiCl-PrCl3, J. Alloy Compd., 2005, 396, p 92-99
H.J. Seifert, H. Fink, and J. Uebach, Properties of Double Chlorides in the Systems ACl/NdCl3 (A: Na-Cs), J. Therm. Anal., 1988, 33, p 625-632
Y. Hosoya, T. Terai, S. Tanaka, and Y. Takahashi, Phase Equilibria of NdCl3-NaCl-KCl, J. Nucl. Mater., 1997, 247, p 304-308
M. Gaune-Escard, L. Rycerz, W. Szczepaniak, and A. Bogacz, Entropies of Phase Transions in the M3LnCl6 Compounds (M ≡ K, Rb, Cs; Ln ≡ La, Cd, Pr, Nd) and K2NdCl5, J. Alloy Compd., 1994, 204, p 189-192
G.W.H. Hohne, W.F. Hemminger, and H.-J. Flammersheim, Differential Scanning Calorimetry, Springer, Berlin Heidelberg, 2003
K. Nakamura and M. Kurata, Thermal Analysis of Pseudo-Binary System: LiCl-KCl Eutectic and Lanthanide Chloride, J. Nucl. Mater., 1997, 247, p 309-314
S. Ghosh, R. Ganesan, R. Sridharan, and T. Gnanasekaran, Measurements of Chemical Activities of Rare Earths (RE: Ce, Pr, Sm and Eu) in Cadmium Alloy, J. Nucl. Mater., 2015, 467, p 280-285
O. Knacke, O. Kubaschewski, and K. Hesselmann, Thermochemical Properties of Inorganic Substances, 2nd ed., Springer, Berlin, 1991
E.M. Levin and H.F. McMurdie, Phase Diagram for Ceramists 1975 Supplement, American Ceramic Society, Columbus, 1975
R.A. Sharma and R.A. Rogers, Phase Equilibria and Structural Species in NdCl3-NaCl, NdCl3-CaCl2, PrCl3-NaCl, and PrCl3-CaCl2 Systems, J. Am. Ceram. Soc., 1992, 75, p 2484-2490
F.N. Rhines, Phase Diagrams in Metallurgy: Their Development and Application, McGraw-Hill Book Company, New York, 1956
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghosh, S., Ganesan, R., Sridharan, R. et al. Investigation on the Phase Diagram of LiCl-KCl-NdCl3 Pseudo-Ternary System. J. Phase Equilib. Diffus. 39, 916–932 (2018). https://doi.org/10.1007/s11669-018-0695-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-018-0695-3