Abstract
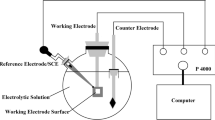
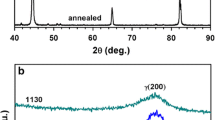
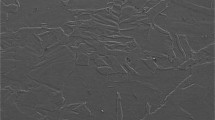
In this study, two different grades (M23 and M29) of cobalt-free low nickel maraging steel have been produced through electroslag remelting (ESR) process. The corrosion resistance of these ESR steels was investigated in 1 M H2SO4 solution using linear potentiodynamic polarization (LPP) and electrochemical impedance spectroscopy (EIS) techniques. The experiments were performed for different immersion time and solution temperature. To evaluate the corrosion resistance of the ESR steels, some significant characterization parameters from LPP and EIS curves were analyzed and compared with that of conventional C250 maraging steel. Irrespective of measurement techniques used, the results show that the corrosion resistance of the ESR steels was higher than the C250 steel. The microstructure of ESR steels was composed of uniform and well-distributed martensite accompanied with little amount of retained austenite in comparison with C250 steel.









Similar content being viewed by others
References
J. Razek, I.E. Klein, and J. Yahalom, Structure and Corrosion Resistance of Oxides Grown on Maraging Steel in Steam at Elevated Temperatures, Appl. Surf. Sci., 1997, 108, p 159–167
Y. Ohue and K. Matsumoto, Sliding-Rolling Contact Fatigue and Wear of Maraging Steel Roller with Ion-Nitriding and Fine Particle Shot-Peening’, Wear, 2007, 263, p 782–789
W. Wang, W. Yan, Q. Duan, Y. Shan, Z. Zhang, and K. Yang, Study on Fatigue Property of a New 2.8 GPa Grade Maraging Steel, Mater. Sci. Eng. A, 2010, 527, p 3057–3063
T. Poornima, J. Nayak, and A.N. Shetty, Corrosion of Aged and Annealed 18 Ni 250 Grade Maraging Steel in Phosphoric Acid Medium, Int. J. Electrochem. Sci., 2010, 5, p 56–71
R.F. Decker and S. Floreen, Intern. Symp. on Maraging Steels Recent Development and Applications, vol. 1 (Pheonix, Arizona, 1998), p. 1–88.
K. Stiller, F. Danoix, and A. Bostel, Investigation of Precipitation in a New Maraging Stainless Steel, Appl. Surf. Sci., 1996, 94, p 326–333
D. Klobcar, J. Tusek, B. Taljat, L. Kosec, and M. Pleterski, Aging of Maraging Steel Welds During Aluminum Alloy Die Casting, Mater. Sci., 2008, 44, p 515–522
O. Dmitrieva et al., Chemical Gradients Across Phase Boundaries Between Martensite and Austenite in Steel Studied by Atom Probe Tomography and Simulation, Acta Mater., 2008, 59, p 364–374
J. Grum and J.M. Slabe, Effect of Laser-Remelting of Surface Cracks on Microstructure and Residual Stresses in 12Ni Maraging Steel, Appl. Surf. Sci., 2006, 252, p 4486–4492
R.F. Decker, J.T. Eash, and A.J. Goldman, 18% Nickel Maraging Steel, ASM Trans. Quart., 1962, 55, p 58
I.V. Pestov, A. Maloletnev, M.D. Ya Peraks, and N.K. Leonova, Metall. Term. Obrab. Met., 1983, 4, p 38
S. Floreen, Cobalt-Free maraging Steels, H. S. Pat. No. 4443, 1984, p 254.
D.M. Vanderwalker, In Maraging Steels—Recent Developments and Applications, TMS-AIME, Warrendale, PA, 1988, p 255–268
H. Halfa, A. Fathy, M. Kamal, M. Eissa and K. El-Fawahkry, Enhancement of mechanical properties of developed Ti-containing Co-free low Ni maraging steel by ESR, STEEL GRIPS 8, 2010, No. Product& Quality, p. 278/284.
H. Halfa and A.M. Reda, Electroslag Remelting of High Technological Steels, J. Miner. Mater. Charact. Eng., 2015, 3, p 444–457
G. Bellanger and J.J. Rameau, Effect of Slightly Acid pH with or Without Chloride in Radioactive Water on the Corrosion of Maraging Steel, J. Nucl. Mater., 1996, 228, p 24–37
G. Bellanger, Effect of Carbonate in Slightly Alkaline Medium on the Corrosion of Maraging Steel, J. Nucl. Mater., 1994, 217, p 187–193
J. Rezek, I.E. Klein, and J. Yhalom, Electrochemical Properties of Protective Coatings on Maraging Steel, Corros. Sci., 1997, 39, p 385–397
T. Poornima, N. Jagannatha, and A.N. Nityananda, Studies on Corrosion of Annealed and Aged 18 Ni 250 Grade Maraging Steel in Sulphuric Acid Medium, Portugal, Electrochim. Acta, 2010, 28, p 173–188
H. Ono, K. Morita, and N. Sano. Metallurg. Mat. Trans. 266B (1995), p 991/96.
J. H. Papier, A Stainless Maraging Steel Used in Aerospace Application, The Minerals, Metals & Materials Society, Edited by Richard K. Wilson (1988), p. 125–156.
J.M. Pardal, S.S.M. Tavares, V.F. Terra, M.R. Da Silva, and D.R. Dos Santos, Modeling of Precipitation Hardening During the Aging and Overaging of 18Ni-Co-Mo-Ti Maraging 300 Steel, J. Alloys Compd., 2005, 393, p 109–113
E.J. Mittemeijer, A. van Gent, and P.J. van der Schaaf, Analysis of Transformation Kinetics by Nonisothermal Dilatometry, Metal. Trans., 1986, 17A, p 1441–1445
P.P. Sinha, D. Sivakumar, N.S. Babu, K.T. Tharian, and A. Natarajan, Austenite Reversion in 18 Ni Co-Free Maraging Steel, Steel Res., 1995, 11, p 490
E.-S.M. Sherif, Corrosion Behavior of Duplex Stainless Steel Alloy Cathodically Modified with Minor Ruthenium Additions in Concentrated Sulfuric Acid Solutions, Int. J. Electron. Sci., 2011, 6, p 2284–2298
A.K. Singh, S.K. Shukla, M. Singh, and M.A. Quraishi, Inhibitive Effect of Ceftazidime on Corrosion of Mild Steel in Hydrochloric Acid Solution, Mater. Chem. Phys., 2011, 129, p 68–76
M. El Azhar, B. Mernari, M. Traisnel, F. Bentiss, and M. Lagrenée, Corrosion Inhibition of Mild Steel by the New Class of Inhibitors [2,5-bis(n-Pyridyl)-1,3,4-thiadiazoles] in Acidic Media, Corros. Sci., 2001, 43, p 2229–2238
J.R. Macdonald and W.B. Johnson, Theory in Impedance Spectroscopy, Wiley, New York, 1987
H. Wang, X. Wang, H. Wang, L. Wang, and A. Liu, DFT Study of New Bipyrazole Derivatives and Their Potential Activity as Corrosion Inhibitors, J. Mol. Model., 2007, 13, p 147–153
L. Larabi, Y. Harek, O. Benali et al., Hydrazide Derivatives as Corrosion Inhibitors for Mildsteel in 1 M HCl, Prog. Org. Coat., 2005, 54, p 261
Acknowledgments
The author extends his appreciation to the Center of Excellence for Research in Engineering Materials (CEREM) of Advanced Manufacturing Institute, King Saud University, Riyadh, Saudi Arabia for funding the work. The steel samples used in this study have been produced and refined at the pilot plant of Steel and Ferroalloys Department, Central Metallurgical Research and Development Institute “CMRDI,” Egypt.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seikh, A.H., Halfa, H., Baig, M. et al. Microstructure Characterization and Corrosion Resistance Behavior of New Cobalt-Free Maraging Steel Produced Through ESR Techniques. J. of Materi Eng and Perform 26, 1589–1597 (2017). https://doi.org/10.1007/s11665-017-2568-z
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-017-2568-z