Abstract
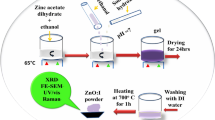
Fe-doped TiO2 powders were synthesized by the sol–gel method using titanium (IV) isopropoxide (TTIP) as the starting material, ethanol as solvent, and ethylene glycol (EG) as stabilizer. These prepared samples were characterized by x-ray diffractometer (XRD), field emission scanning electron microscope (FESEM), Fourier-transform infrared (FTIR) spectroscopy, diffuse reflection spectroscopy (DRS), energy-dispersive x-ray spectroscopy (EDX), and photoluminescence (PL) analyses to study their structure, morphology, and optical properties. The particle size of Fe-doped TiO2 was in the range of 18–39 nm and the minimum crystallite size was achieved for 4 mol.% of Fe. The XRD result of the samples that were doped with Fe showed a tetragonal structure. It also revealed the coexistence of the anatase and rutile phases, and showed that their ratio changed with various molar concentrations of Fe dopant. FTIR spectroscopy showed the presence of the Ti-O vibration band in the samples. PL analysis revealed the PL property in the UV region. Visible irradiation and the intensity of PL spectra were both reduced by doping TiO2 with 3 mol.% of Fe as compared to the pure variety. The spectra from the DRS showed a red shift and a reduction of 2.6 eV in the band gap energy for 4 mol.% Fe-doped TiO2. The optimum level of impurity (4 mol.%) for Fe-doped TiO2 nanoparticles (NPs), which improve the optical and electrical properties by using new precursors and can be used in solar cells and electronic devices, was determined. The novelty of this work consists of: the Fe/TiO2 NPs are synthesized by new precursors from sol–gel synthesis of iron and TTIP using acetic acid-catalyzed solvolysis (original idea) and the optical properties optimized with a mixture of phases (anatase/rutile) of Fe-doped TiO2 by this facile method.
Similar content being viewed by others
References
C.O. Robichaud, A.E. Uyar, M.R. Darby, K.G. Zycker, and M.R. Wiesner, Environ. Sci. Technol. 43, 4227 (2009).
M. Dastpak, M. Farahmandjou, and T.P. Firoozabadi, J. Supercond. Nov. Magn. 29, 2925 (2016).
M. Farahmandjou and M. Zarinkamar, J. Ceram. Process. Res. 17, 166 (2016).
M. Farahmandjou and N. Golabiyan, J. Ceram. Process. Res. 16, 237 (2015).
M. Farahmandjou, S. Honarbakhsh, and S. Behrouzinia, Phys. Chem. Res. 4, 655 (2016).
M. Farahmandjou, M. Zarinkamar, and T.P. Firoozabadi, Rev. Mex. Fis. 62, 76 (2016).
M. Dastpak, M. Farahmandjou, and T.P. Firoozabadi, J. Supercond Nov. Magn. 29, 849 (2016).
M. Farahmandjou and F. Soflaee, Chin. J. Phys. 53, 080801 (2015).
M. Farahmandjou, Acta Phys. Pol. A 123, 277 (2013).
A. Fujishima, T.N. Rao, and D.A. Truk, J. Photochem. Photobiol. C Photochem. Rev. 1, 1 (2000).
X. Chen and S.S. Mao, Chem. Rev. 107, 2891 (2007).
B.M. Reddy, I. Ganesh, and A. Khan, J. Mol. Catal. A Chem. 223, 295 (2004).
K. Josep Antony Raj and B. Vishwanathan, Indian J. Chem. 48, 1378 (2009).
L. Gang, W. Xuewen, C. Zhigang, C. Hui-Ming, and L.G. Qing, Colloid Interface Sci. 329, 331 (2009).
M. Ramazani, M. Farahmandjou, and T.P. Firoozabadi, Phys. Chem. Res. 3, 293 (2015).
M. Ramazani, M. Farahmandjou, and T.P. Firoozabadi, Int. J. Nanosci. Nanotechnol. 11, 115 (2015).
J. Yu, Q. Xiang, and M. Zhou, Appl. Catal. B Environ. 90, 595 (2009).
U.G. Akpan and B.H. Hameed, Appl. Catal. A 375, 1 (2010).
W.Y. Choi, A. Termin, and M.R. Hoffmann, J. Phys. Chem. 98, 13669 (1994).
R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki, and Y. Taga, Science 293, 269 (2011).
J. Choi, H. Park, and M. Hoffmann, J. Phys. Chem. C 114, 783 (2010).
A. Kachina, E. Puzenat, S. Ould-Chikh, C. Geantet, P. Delichere, and P. Afanasiev, Chem. Mater. 24, 636 (2012).
J. Zhang, C. Pan, P. Fang, J. Wei, R. Xiong, and A.C.S. Appl, Mater. Interfaces 2, 1173 (2010).
H. Liu, Y. Wu, J. Zhang, and A.C.S. Appl, Mater. Interfaces 3, 1757 (2011).
D.R. Pulsipher, I.T. Martin, E.R. Fisher, and A.C.S. Appl, Mater. Interfaces 2, 1743 (2010).
M.A.T. Izmajlowicz, A.J. Flewitt, W.I. Milne, and N.A. Morrison, J. Appl. Phys. 94, 7535 (2003).
M. Epifani, C. Giannini, L. Tapfer, and L. Vasanelli, J. Am. Ceram. Soc. 83, 2385 (2000).
B. Khoshnevisan, M.B. Marami, and M. Farahmandjou, Chin. Phys. Lett. 35, 027501 (2018).
N. Bouazizi, R. Bargougui, T. Boudharaa, M. Khelil, A. Benghnia, L. Labiadh, R.B. Slama, B. Chaouachi, S. Ammar, and A. Azzouz, Ceram. Int. 42, 9413 (2016).
N. Bouazizi, F. Ajala, M. Khelil, H. Lachheb, K. Khirouni, A. Houas, and A. Azzouz, J. Mater. Sci. Mater. Electron. 27, 11168 (2016).
I. Ganesh, P. Kumar, K. Gupta, S.C. Panakati, R. Kalathur, P. Gadhe, and S. Govindan, Proc. Appl. Ceram. 6, 21 (2012).
H. Yamashita, M. Harada, J. Misaka, M. Takeuchi, B. Neppolian, and M. Anpo, Catal. Today 84, 191 (2003).
X.H. Wang, J.-G. Li, H. Kamiyama, and T. Ishigaki, Thin Solid Films 278, 506 (2006).
C.C. Trapalis, P. Keivanidis, G. Kordas, M. Zaharescu, M. Crisan, A. Szatvanyi, and M. Gartner, Thin Solid Films 433, 186 (2003).
M. Sokmen, F. Candan, and Z. Sumer, J. Photochem. Photobiol. Chem. 143, 241 (2001).
I. Djerdj and A.M. Tonejc, J. Alloy Compd. 413, 159 (2006).
J. Moser, M. Gratzel, and R. Gallay, Helv. Chim. Acta 70, 1596 (1987).
W. Li, A.I. Frenkel, J.C. Woicik, C. Ni, and S.I. Shah, Phys. Rev. B 72, 155315 (2005).
R.S. Santos, G.A. Faria, C. Giles, C.A.P. Leite, H.S. Barbosa, M.A.Z. Arruda, C. Longo, and A.C.S. Appl, Mater. Interfaces 4, 5555 (2012).
D. Reyes-corondo, G. Rodriguez-gattorno, M.E. Espinosa-Pesqueira, C. Cab, R. de Coss, and G. Oskam, Nanotechnology 19, 145605 (2008).
Y.H. Zhang and A. Reller, J. Mater. Chem. 11, 2537 (2001).
J. Zhu, W. Zheng, B. He, J. Zhang, and M. Anpo, J. Mol. Catal A 216, 35 (2004).
C.Y. Wang, C. Bottcer, D.W. Bahnemann, and J.K. Dohrmann, J. Mater. Chem. 13, 2322 (2003).
M. Hiran, T. Joji, M. Inagaki, and H. Iwata, J. Am. Ceram. Soc. 87, 35 (2004).
R. Alexandrescu, I. Morjan, M. Scarisoreanu, R. Birjega, E. Popovici, I. Soare, L. Gavrila-Florescu, I. Voicu, I. Sandu, F. Dumitrache, G. Prodan, E. Vasile, and E. Figgemeier, Thin Solid Films 515, 8438 (2007).
X. Zhang, M. Zhou, and L. Lei, Catal. Commun. 7, 427 (2006).
S. Liu, X. Liu, Y. Chen, and R. Jiang, J. Alloys Compd. 506, 877 (2010).
S. Reginaldo, S. Santos, A. Guilherme, A.P. Carlos, S. Leite, S. Herbert, A.Z. Marco, C. Longo, and A.C.S. Appl, Mater. Interfaces 4, 5555 (2012).
T. Ali, P. Tripathi, A. Azam, W. Raza, A.S. Ahmed, A. Ahmed, and M. Muneer, Mater. Res. Express 4, 015022 (2017).
Y. Yang, T. Yu, J. Wang, W. Zheng, and Y. Cao, Cryst. Eng. Comm. 19, 1100 (2017).
R.J. Ramalingam, P. Arunachalam, T. Radhika, K.R. Anju, K.C. Nimitha, and H.A. Al-Lohedan, Int. J. Electrochem. Sci. 12, 797 (2017).
C.L. Luu, Q.T. Nguyen, and S.T. Ho, Adv. Nat. Sci. Nanosci. Nanotechnol 1, 015008 (2010).
A.R. Denton and N.W. Ashcroft, Phys. Rev. A 43, 3161 (1991).
Q. Chen, C. Xue, X. Li, and Y. Wang, Mater. Sci. Forum 743, 367 (2013).
S. Khatoon, I.A. Wani, J. Ahmed, T. Magdaleno, O.A. Al-Hartomy, and T. Ahmad, Mater. Chem. Phys. 138, 519 (2013).
W. Siripala and M. Tomkieviez, J. Electrochem. Soc. 129, 1240 (1982).
J. Schneider, M. Matsuoka, M. Takeuchi, J. Zhang, Y. Horiuchi, M. Anpo, and D.W. Bahnemann, Chem. Rev. 114, 9919 (2014).
N.D. Abazovic, M.I. Comor, M.D. Dramicanin, D.J. Jovanovic, S.P. Ahrenkiel, and J.M. Nedeljkovic, J. Phys. Chem. B 110, 25366 (2006).
N.D. Abazovic´, I.A. Ruvarac-Bugarcˇic´, M.I. Comor, N. Bibic´, S.P. Ahrenkiel, and J.M. Nedeljkovic´, Opt. Mater. 30, 1139 (2008).
D. Beydoun, R. Amal, G. Low, and S. McEvoy, J. Nanopart. Res. 1, 439 (1999).
L. Kernazhitsky, V. Shymanovska, V. Naumov, V. Chernyak, T. Khalyavka, and V. Kshnyakin, Ukr. J. Phys. Opt. 9, 197 (2008).
W. Zhao, W. Fu, H. Yang, C. Tian, M. Li, J. Ding, X. Zhou, H. Zhao, Y. Li, and W. Zhang, Nano Micro Lett. 3, 34 (2011).
S. Naghibia, S. Vahed, and O. Torabi, J. Adv. Mater. Process. 2, 55 (2014).
W. Zhao, W. Fu, H. Yang, C. Tian, M. Li, J. Ding, W. Zhang, X. Zhou, H. Zhao, and Y. Li, Nano-Micro Lett. 3, 20 (2011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marami, M.B., Farahmandjou, M. & Khoshnevisan, B. Sol–Gel Synthesis of Fe-Doped TiO2 Nanocrystals. J. Electron. Mater. 47, 3741–3748 (2018). https://doi.org/10.1007/s11664-018-6234-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-018-6234-5