Abstract
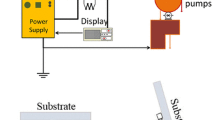
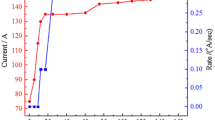
In the current study, composites of tungsten trioxide (W03) and silver (Ag) are deposited in a layer-by-layer electrochromic (EC) arrangement onto a fluorine-doped tin oxide coated glass substrate. Tungsten oxide nanoparticles are an n-type semiconductor that can be used as EC cathode material. Nano-sized silver is a metal that can serve as an electron trap center that facilitates charge departure. In this method, the WO3 and Ag nanoparticle powder were deposited by physical vapor deposition onto the glass substrate. The fabricated electrochromic devices (ECD) were post-annealed to examine the effect of temperature on their EC properties. The morphology of the thin film was characterized by scanning electron microscopy and atomic force microscopy. Structural analysis showed that the addition of silver dopant increased the size of the aggregation of the film. The film had an average approximate roughness of about 17.8 nm. The electro-optical properties of the thin film were investigated using cyclic voltammetry and UV–visible spectroscopy to compare the effects of different post-annealing temperatures. The ECD showed that annealing at 200°C provided better conductivity (maximum current of about 90 mA in the oxidation state) and change of transmittance (ΔT = 90% at the continuous switching step) than did the other thin films. The optical band gaps of the thin film showed that it allowed direct transition at 3.85 eV. The EC properties of these combinations of coloration efficiency and response time indicate that the WO3-Ag-WO3-Ag arrangement is a promising candidate for use in such ECDs.
Similar content being viewed by others
References
P. Yang, P. Sun, and W. Mai, Mater. Today 19, 394 (2016).
S. Hoseinzadeh, A. Bahari, R. Ghasemiasl, and A.H. Ramezani, J. Mater. Sci. Mater. Electron. 28, 14855 (2017).
S. Hoseinzadeh, A. Bahari, R. Ghasemiasl, and A.H. Ramezani, J. Mater. Sci. Mater. Electron. 28, 14446 (2017).
H. Najafi-Ashtiani, A. Bahari, S. Gholipour, and S. Hoseinzadeh, Appl. Phys. A 124, 24 (2018).
C.G. Granqvist, S. Green, G.A. Niklasson, N.R. Mlyuka, S. von Kræmer, and P. Georén, Thin Solid Films 518, 3046 (2010).
V.R. Buch, A.K. Chawla, and S.K. Rawal, Mater. Today Proc. 3, 1429 (2016).
N. Tripathi, L.D. Bell, S. Nikzad, M. Tungare, P.H. Suvarna, and F.S. Sandvik, J. Electron. Mater. 40, 382 (2011).
A. Bahari and M. Shahbazi, J. Electron. Mater. 45, 1201 (2016).
H. Najafi-Ashtiani, A. Bahari, and S. Ghasemi, Organ. Electron. 37, 213 (2016).
P. Kumar, K.S. Narayan, S. Guha, and F. Shahedipour-Sandvik, Organ. Electron. 14, 2818 (2013).
R. Gholipur, Z. Khorshidi, and A. Bahari, ACS Appl. Mater. Interfaces 9, 12528 (2017).
F. Shahedipour-Sandvik and B.W. Wessels, Appl. Phys. Lett. 76, 3011 (2000).
X.A. Cao, K. Topol, F. Shahedipour-Sandvik, J. Teetsov, P.M. Sandvik, S.E. LeBoeuf, A. Ebong, J.W. Kretchmer, E.B. Stokes, S. Arthur, and A.E. Kaloyeros, in Proceedings of SPIE 4776, Solid State Lighting II (2002)
C.P. Cheng, Y. Kuo, C.P. Chou, C.H. Cheng, and T.P. Teng, Appl. Phys. A Mater. Sci. Process. 116, 1553 (2014).
K.J. Patel, C.J. Panchal, M.S. Desai, and P.K. Mehta, Mater. Chem. Phys. 124, 884 (2010).
R. Baetens, B.P. Jelle, and A. Gustavsen, Sol. Energy Mater. Sol. Cells 94, 87 (2010).
Y. Guo, X. Quan, N. Lu, H. Zhao, and S. Chen, Environ. Sci. Technol. 41, 4422 (2007).
I.C. Amaechi, A.C. Nwanya, P.U. Asogwa, R.U. Osuji, M. Maaza, and F.I. Ezema, J. Electron. Mater. 44, 1110 (2015).
A.H. Ramezani, S. Hoseinzadeh, and A. Bahari, J. Inorg. Organomet. Polym. First Online: 02 January (2018).
C.G. Granqvist, Sol. Energy Mater. Sol. Cells 99, 1 (2012).
V.V. Ganbavle, J.H. Kim, and K.Y. Rajpure, J. Electron. Mater. 44, 874 (2015).
S.-H. Park, S.-M. Lee, E.-H. Ko, T.-H. Kim, Y.-C. Nah, S.-J. Lee, J.H. Lee, and H.-K. Kim, Sci. Rep. 6, 33868 (2016).
R.R. Kharade, S.S. Mali, S.P. Patil, K.R. Patil, M.G. Gang, P.S. Patil, J.H. Kim, and P.N. Bhosale, Electrochim. Acta 102, 358 (2013).
H. Li, Y. Lv, X. Zhang, X. Wang, and X. Liu, Sol. Energy Mater. Sol. Cells 136, 86 (2015).
K.W. Park, Electrochim. Acta 50, 4690 (2005).
H. Huang, J. Tian, W.K. Zhang, Y.P. Gan, X.Y. Tao, X.H. Xia, and J.P. Tu, Electrochim. Acta 56, 4281 (2011).
C. Feng, S. Wang, and B. Geng, Nanoscale 3, 3699 (2011).
D. Dastan, S.L. Panahi, and N.B. Chaure, J. Mater. Sci. Mater. Electron. 27, 12291 (2016).
D. Dastan and A. Banpurkar, J. Mater. Sci. Mater. Electron. 28, 3851 (2016).
S.B. Upadhyay, R.K. Mishra, and P.P. Sahay, Ceram. Int. 42, 15601 (2016).
M. Reghima, A. Akkari, C. Guasch, and N. Kamoun-Turki, J. Electron. Mater. 44, 4392 (2015).
S. Karthika, V. Prathibha, M.K.A. Ann, V. Viji, P.R. Biju, and N.V. Unnikrishnan, J. Electron. Mater. 43, 447 (2014).
V. Vidyadharan, P. Vasudevan, S. Karthika, C. Joseph, N.V. Unnikrishnan, and P.R. Biju, J. Electron. Mater. 44, 2754 (2015).
H. Wei, J. Zhu, S. Wu, S. Wei, and Z. Guo, Polymer (United Kingdom) 54, 1820 (2013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoseinzadeh, S., Ghasemiasl, R., Bahari, A. et al. Effect of Post-annealing on the Electrochromic Properties of Layer-by-Layer Arrangement FTO-WO3-Ag-WO3-Ag. J. Electron. Mater. 47, 3552–3559 (2018). https://doi.org/10.1007/s11664-018-6199-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-018-6199-4