Abstract
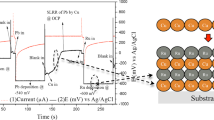
The effects of additives to an acidic electrolyte for electrochemical deposition of copper film to prevent corrosion of the Co/SiO2/Si substrate have been investigated. A sacrificial Pb layer was formed by underpotential deposition (UPD), then a Cu layer was prepared using surface-limited redox replacement (SLRR) to exchange the UPD-Pb layer in an acidic copper electrolyte with trisodium citrate, sodium perchlorate, and ethylenediamine as additives. The additives significantly affected the replacement of UPD-Pb by Cu and prevented galvanic corrosion of the Co/SiO2/Si substrate in the acidic Cu electrolyte. The results showed that both sodium perchlorate and ethylenediamine reduced the corrosion of the Co substrate and resulted in Cu film with low electrical resistivity. However, residual Pb was present in the Cu film when using trisodium citrate, as the citrate ions slowed copper displacement. The proposed sequential UPD-Pb and SLRR-Cu growth method may enable electrochemical deposition for fabrication of Cu interconnects on Co substrate from acidic Cu electrolyte.
Similar content being viewed by others
References
S.M. George, Chem. Rev. 110, 111 (2010).
Y. Shacham-Diamand, V. Dubin, and M. Angyal, Thin Solid Films 262, 93 (1995).
M. Paunovic, P.J. Bailey, R.G. Schand, and D.A. Smith, J. Electrochem. Soc. 141, 1843 (1994).
S.T. Chen, Y.Y. Liu, and G.S. Chen, Appl. Surf. Sci. 354, 144 (2015).
H.B. Bhandari, J. Yang, H. Kim, Y. Lin, R.G. Gordon, Q.M. Wang, J.S.M. Lehn, H. Li, and D. Shenai, ECS J. Solid State Sci. Technol. 1, N79 (2012).
Y.S. Diamand, B. Israel, and Y. Sverdlov, Microelectron. Eng. 55, 313 (2001).
A. Kohn, M. Eizenberg, Y.S. Diamand, B. Israel, and Y. Sverdlov, Microelectron. Eng. 55, 297 (2001).
T.K. Tsai, S.S. Wu, W.L. Liu, S.H. Hsieh, and W.J. Chen, J. Electron. Mater. 36, 1408 (2007).
T. Osaka, H. Aramaki, M. Yoshino, K. Ueno, I. Matsuda, and Y.S. Diamand, J. Electrochem. Soc. 156, H707 (2009).
T.K. Tsai, S.S. Wu, C.S. Hsu, and J.S. Fang, Thin Solid Films 519, 4958 (2011).
H. Einati, V. Bogush, Y. Sverdlov, Y. Rosenberg, and Y.S. Diamand, Microelectron. Eng. 82, 623 (2005).
W.Z. Xu, J.X. Wang, H.S. Lu, X. Zeng, J.B. Xu, and X.P. Qu, in IEEE ICSICT Conference Proceeding (2012), pp. 1–3.
J. Gong, I. Zana, and G. Zangari, J. Mater. Sci. Lett. 20, 1921 (2001).
P. Wei, O.E. Jileman, M.R. Bateni, X. Deng, and A. Petric, Surf. Coat. Technol. 201, 7739 (2007).
H.S. Lu, J.X. Wang, X. Zeng, F. Chen, X.M. Zhang, W.J. Zhang, and X.P. Qu, Electrochem. Solid-State Lett. 15, H97 (2012).
V. Brusic, G.S. Frankel, A.G. Schrott, T.A. Petersen, and B.M. Rush, J. Electrochem. Soc. 140, 2507 (1993).
B.C. Peethala, H.P. Amanapu, U.R.K. Lagudu, and S.V. Babu, J. Electrochem. Soc. 159, H582 (2012).
The International Technology Roadmap for Semiconductors 2.0, 2015 edition, Interconnect, http://www.itrs2.net/itrs-reports.html.
J.S. Fang, Y.S. Liu, and T.S. Chin, Thin Solid Films 480, 1 (2015).
J.S. Fang, S.L. Sun, Y.L. Cheng, G.S. Chen, and T.S. Chin, Appl. Surf. Sci. 364, 358 (2016).
T.R.I. Cataldi and G.E. De Benedetto, J. Electroanal. Chem. 458, 149 (1998).
C. Thambidurai, Y.G. Kim, N. Jayaraju, V. Venkatasamy, and J.L. Stickney, J. Elecrochem. Soc. 156, D261 (2009).
J.Y. Kim, Y.G. Kim, and J.L. Stickney, J. Electroanal. Chem. 621, 205 (2008).
A. Radisic, Y. Cao, P. Taephaisitphongse, A.C. West, and P.C. Searson, J. Electrochem. Soc. 150, C362 (2003).
J.S. Fang, J.H. Chen, G.S. Chen, Y.L. Cheng, and T.S. Chin, Electrochim. Acta 206, 45 (2016).
R. Vasilic, N. Vasiljevic, and N. Dimitrov, J. Electroanal. Chem. 580, 203 (2005).
G.M. Brisard, E. Zenati, H.A. Gasteiger, N.M. Markovic, and P.N. Ross, Langmuir 11, 2221 (1995).
Y.Z. Hamada, R. Cox, and H. Hamada, J. Basic Appl. Sci. 11, 583 (2015).
J.L. Stickney, C. Thambidurai, and Y.G. Kim, Electrochim. Acta 53, 6157 (2008).
A. Eftekhari, Microelectron. Eng. 69, 17 (2003).
L. Graham, C. Steinbrüchel, and D.J. Duquette, J. Electrochem. Soc. 149, C390 (2002).
M.O. Finot, G.D. Braybrook, and M.T. McDermott, J. Electroanal. Chem. 466, 234 (1999).
B. Scharifker and G. Hills, Electrochim. Acta 28, 879 (1983).
X.D. Gao, X.M. Li, and W.D. Yu, J. Solid State Chem. 178, 1139 (2005).
W.Z. Xu, J.B. Xu, H.S. Lu, J.X. Wang, Z.J. Hu, and X.P. Qu, J. Electrochem. Soc. 160, D3075 (2013).
B.J. Hwang, R. Santhanam, and Y.L. Lin, Electrochim. Acta 46, 2843 (2001).
Y.G. Kim, J.Y. Kim, D. Vairavapandian, and J.L. Stickney, J. Phys. Chem. B 110, 17998 (2006).
C. Thambidurai, D.K. Gegregziabiher, X. Liang, Q. Zhang, V. Ivanova, P.H. Haumesser, and J.L. Stickney, J. Electrochem. Soc. 157, D466 (2010).
L.B. Sheridan, D.K. Gebregziabiher, J.L. Stickney, and D.B. Robinson, Langmuir 29, 1592 (2013).
S. Kim and D.J. Duquette, J. Electrochem. Soc. 153, C417 (2006).
S. Aksu and F.M. Doyle, J. Electrochem. Soc. 149, B340 (2002).
Acknowledgements
The authors acknowledge financial support provided by the Ministry of Science and Technology, Taiwan under Grant 104-2221-E-150-005-MY2 and experimental support provided by the Common Laboratory for Micro/Nano Science and Technology of the National Formosa University for this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fang, J.S., Lin, L.Y., Wu, C.L. et al. Effects of Additives on Electrochemical Growth of Cu Film on Co/SiO2/Si Substrate by Alternating Underpotential Deposition of Pb and Surface-Limited Redox Replacement by Cu. J. Electron. Mater. 46, 6677–6684 (2017). https://doi.org/10.1007/s11664-017-5692-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-017-5692-5