Abstract
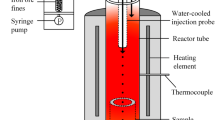
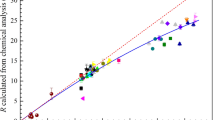
The ironmaking processes that directly use iron ore fines as raw material are under development and receiving more and more attention. In a flash reduction process, both the thermal decomposition reaction and the reduction reaction of ore fines are extremely fast and cause loss of oxygen from iron oxides. However, it is difficult to distinguish between the thermal decomposition and reduction during the conversion from hematite to magnetite. In this work, the thermal decomposition behavior of hematite ore fines with different particle sizes is investigated by using a thermogravimetric analyzer (TGA). The kinetic parameters are calculated based on the Coats–Redfern method and then verified by the Satava–Sestak method. The F2 model is identified as the most probable mechanism function under the present experimental conditions. The average values of activation energy and the pre-exponential factor are 1256 kJ mol−1 and 1.94 × 1041 s−1, respectively. The internal morphology of the fine hematite particle with partial decomposition is observed to further investigate the reaction mechanism. Moreover, the relative contribution of the two kinds of chemical reactions (thermal decomposition and gaseous reduction) to the overall conversion process from hematite to magnetite is investigated by kinetic calculations based on the obtained reaction rate equations.












Similar content being viewed by others
References
Y. Nakano, M. Ishida, T. Akehata, and T. Shirai: Metall. Trans. B, 1975, vol. 6B, pp. 429–34.
S. Hayashi and Y. Iguchi: ISIJ Int., 1990, vol. 30, pp. 722–30.
A. Orth, N. Anastasijevic, and H. Eichberger: Miner. Eng., 2007, vol. 20, pp. 854–61.
M.A. Quader, S. Ahmed, S.Z. Dawal, and Y. Nukman: Renew. Sustain. Energy Rev., 2016, vol. 55, pp. 537–49.
H.K. Pinegar, M.S. Moats, and H.Y. Sohn: Ironmaking Steelmaking, 2012, vol. 39, pp. 398–06.
H.K. Pinegar, M.S. Moats, and H.Y. Sohn: Ironmaking Steelmaking, 2013, vol. 40, pp. 32–43.
H. Wang and H.Y. Sohn: Steel Res. Int., 2012, vol. 83, pp. 903–09.
H. Wang and H.Y. Sohn: Metall. Mater. Trans. B, 2013, vol. 44B, pp. 1133–45.
F. Chen, Y. Mohassab, T. Jiang, and H.Y. Sohn: Metall. Mater. Trans. B, 2015, vol. 46B, pp. 1133–45.
A. Habermann, F. Winter, H. Hofbauer, J. Zirngast, and J. L. Schenk: ISIJ Int., 2000, vol. 40, pp. 935–42.
H. Fan, B. Son, and Q. Li: Mater. Chem. Phys., 2006, vol. 98, pp. 148–53.
O. Ozdemir and D.J. Dunlop: Earth Plan. Sci. Lett., 2000, vol. 177, pp. 59–67.
L.X. Yang and S. Witchard: ISIJ Int., 1998, vol. 38, pp. 1069–76.
E.T. Turkdogan and J.V. Vinters: Metall. Trans., 1971, vol. 2, pp. 3175–88.
E.T. Turkdogan and J.V. Vinters: Metall. Trans., 1971, vol. 2, pp. 3189-96.
Y. X. Qu, Y.X. Yang, Z.S. Zou, C. Zeilstra, K. Meijer, and R. Boom: ISIJ Int., 2015, vol. 55, pp. 952–60.
M. Salmani, E.K. Alamdari, and S. Firoozi: J. Therm. Anal. Calorim., 2017, vol. 128, pp. 1385–90.
H.M. Ahmed, P. Semberg, C. Andersson, and B. Bjorkman: ISIJ Int., 2018, vol. 58, pp. 446–52.
M. Sorescu and T. Xu: J. Therm. Anal. Calorim., 2012, vol. 107, pp. 463–69.
M. Sorescu, T. Xu, and L. Diamandescu: Mater. Charact., 2010, vol. 61, pp. 1103–18.
M. Sorescu and L. Diamandescu: Hyperfine Interact., 2010, vol. 196, pp. 349–58.
Y.X. Qu, Y.X. Yang, Z.S. Zou, C. Zeilstra, K. Meijer, and R. Boom: ISIJ Int., 2014, vol. 54, pp. 2196–05.
X. Zhang, Y. Han, Y. Li, and Y. Sun: Mineral, 2017, vol. 7, pp. 211–24.
A.W. Coats and J.P. Redfern: Nature, 1964, vol. 201, pp. 68–69.
V. Satava and J. Sestak: J. Therm. Anal., 1975, vol. 8, pp. 477–89.
P.C. Beuria, S.K. Biswal, B.K. Mishra, and G.G. Roy: Int. J. Miner. Metall. Mater. 2017, vol. 24, pp. 229–39.
A.K. Galwey and M.E. Brown: Thermochimica Acta, 1995, vol. 269/270, pp. 1–25.
A. Khawam and D.R. Flanagan: J. Pharm. Sci., 2006, vol. 95, pp. 472–98.
J. Sestak: J. Therm. Anal., 2012, vol. 110, pp. 5–16.
M.E. Brown and A.K. Galwey: Anal. Chem., 1989, vol. 61, pp. 1136–39.
A.K. Galwey and M.E. Brown: J. Therm. Anal. Calorim., 2000, vol. 60, pp. 863–77.
P.K. Strangway: Master’s Thesis, Toronto University, Toronto, ON, Canada, 1964.
L. Xing, Z. Zou, Y. Qu, L. Shao, and J. Zou: Steel Res. Int., 2019. https://doi.org/10.1002/srin.201800311.
Y. Qu, Y. Yang, Z. Zou, C. Zeilstra, K. Meijer, and R. Boom: ISIJ Int., 2015, vol. 55, pp. 149–57.
Acknowledgments
The authors are grateful for the financial support from the National Natural Science Foundation of China (Grant Nos. 51504056, 51604068, and 51574046) and the Fundamental Research Funds for the Central Universities (Grant No. N182504012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted July 12, 2019.
Rights and permissions
About this article
Cite this article
Xing, L., Qu, Y., Wang, C. et al. Kinetic Study on Thermal Decomposition Behavior of Hematite Ore Fines at High Temperature. Metall Mater Trans B 51, 395–406 (2020). https://doi.org/10.1007/s11663-019-01747-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-019-01747-1