Abstract
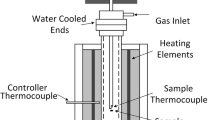
To provide fundamental information on the phases and microstructures formed during sintering, a liquid with a bulk composition within the silico ferrite of calcium (SFC) primary phase field in the ternary “Fe2O3”-CaO-SiO2 system in air was solidified at a controlled rate. Samples of a bulk composition with a CaO/SiO2 ratio of 4.00 and 69.24 wt pct Fe2O3, were cooled from 1623 K (1350 °C) at 2 K/s, with samples quenched at temperatures between 1513 K (1240 °C) to 1453 K (1180 °C). The silico ferrite of calcium and aluminium I (SFCA-I) and Ca7.2Fe 2+0.8 Fe303+O53 phases were observed to form an intergrowth (‘SFC-I’) rather than the anticipated SFC phase. Solidification was found to occur in three stages, Liquid + ‘SFC-I’, Liquid + ‘SFC-I’ + C2S + CF2, and C2S + CF2 + CF, where C2S denotes dicalcium silicate, CF denotes calcium ferrite and CF2 denotes calcium diferrite. The phases formed and the solidification sequence differ from those predicted under equilibrium and Scheil–Gulliver Cooling. Although not directly applicable to industrial operations, this research clearly shows that the formation of both the SFCA and SFCA-I phase in iron ore sinters is controlled by kinetic processes rather than equilibrium conditions.

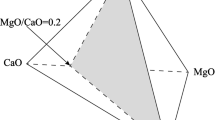
Adapted from Ref. [16]





Adapted from Ref. [16]









Similar content being viewed by others
References
J. Ostwald, BHP Tech. Bull. 1981, vol. 25, pp. 13-20.
W. Mumme, J. Clout and R. Gable, Neues Jahrb. Mineral. Abh. 1998, vol. 173, pp. 93-117.
W.G. Mumme, Neues Jahrb. Miner. Abh., 1988, vol. 8, pp. 359–366.
W.G. Mumme, Neues Jahrb. Mineral. Abh. 2003, vol. 178, pp. 307-335.
E. Grew, U. Halenius, M. Pasero, and J. Barbier, Miner. Mag., 2008, vol. 74, pp. 839–876.
G. Ferraris, E. Makovicky and S. Merlino: Crystallography of Modular Materials. Oxford : Oxford University Press, 2004.
J. Hamilton, B. Hoskins, W. Mumme, W. Borbidge and M. Montague, Neues Jahrb. Miner. Abh. 1989, vol. 161, pp. 1-26.
K. Sugiyama, A. Monkawa and T. Sugiyama, ISIJ Int. 2005, vol. 45, pp. 560-568.
D. Liles, J. de Villiers and V. Kahlenberg, Mineral. Petrol. 2016, vol. 110, pp. 141-147.
T. Patrick and M. I. Pownceby, Metall. Mater. Trans. B 2002, vol. 33, pp. 79-89.
K. Zöll, T. Manninger, V. Kahlenberg, H. Krüger and P. Tropper, Metall. Mater. Trans. B 2017, vol. 48, pp. 2207-2221.
A. Koryttseva, N. Webster, M. Pownceby and A. Navrotsky, J Am Ceram Soc 2017, vol. 100, pp. 3646-3651.
Toru Takayama, Reiko Murao and Masao Kimura, ISIJ International 2018, vol. 58, pp. 1069-1078.
S. Nicol, E. Jak, and P.C. Hayes, Metall. Mater. Trans. B, 2019. https://doi.org/10.1007/s11663-019-01687-w
S. Nicol, E. Jak, J. Chen, W. Qi, X. Mao, and P. Hayes: in The 6th Baosteel Australia Joint Centre Conference 2018, (Wollongong, 2018).
J. Chen, M. Shevchenko, P. Hayes and E. Jak: ISIJ Int., 2019, vol. 59, pp. 795–804.
H.I. Saleh: J. Mater. Sci. Technol., 2004, vol. 20, pp. 530–534.
H. Hughes, P. Roos and D. Goldring, Mineral. Mag. J. Mineral. Soc. 1967, vol. 36, pp. 280-291.
Acknowledgments
The authors would like to thank the Australian Research Council Linkage Program and BHP for financial support to enable this research to be carried out, and the Centre of Microstructure and Microanalysis (CMM), the University of Queensland for providing electron microscope facilities that enabled the microanalytical measurements to be undertaken. This research was supported by an Education Endowment Fund (EEF) scholarship from the Australasian Institute of Mining and Metallurgy (AusIMM) and an Australian Government Research Training Program (RTP) Scholarship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted May 24, 2019.
Rights and permissions
About this article
Cite this article
Nicol, S., Jak, E. & Hayes, P.C. Controlled Solidification of Liquids Within the SFC Primary Phase Field of the “Fe2O3”-CaO-SiO2 System in Air. Metall Mater Trans B 50, 3027–3038 (2019). https://doi.org/10.1007/s11663-019-01689-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-019-01689-8