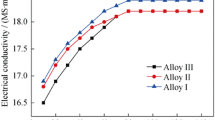
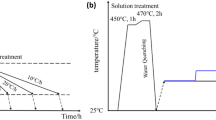
Abstract
To investigate the effects of the quench cooling rate on corrosion resistance of Al-Cu-Mg alloy, an end-quenching test was conducted and the microstructures at different cooling rates were observed by SEM and TEM. Additionally, the corrosion resistance was characterized by an intergranular corrosion test and electrochemical test. Moreover, the finite element method was applied to simulate the end quenching process. The results indicate that the actual end quenching process can be approximated as one-dimensional heat transfer, and the cooling rate varies at different cooling distances. By affecting the microstructures, decreasing the cooling rate leads to a decline in the corrosion properties. Low cooling rates coarsen the constituent particles and grain boundary particles, resulting in a wide precipitation-free zone and an increase in the intensity of corrosion reactions. A high cooling rate concentrates on the intragranular precipitant, which can reduce the pitting depth and represents a conversion from localized corrosion to general corrosion.









Similar content being viewed by others
References
Warner. T: Mater. Sci. Forum, 2006, vol. 519, pp. 1271–78.
T. Dursun, and C. Soutis: Mater. Des., 2014, vol. 56, pp. 862–71.
A. Heinz, A. Haszler, C. Keidel, S. Moldenhauer, R. Benedictus, W. S. Miller: Mater. Sci. Eng. A, 2000, vol. 280, pp. 102-107.
ASTM B209, Standard Specification for Aluminum and Aluminum-Alloy Sheet and Plate, 2014.
Kandpal. B. C, Chutani. A, Gulia. A, and Sadanna. C: International Journal of Advances in Engineering & Technology, 2011, vol. 1, pp. 65.
Cavazos. J. L, and Colás. R: Mater. Sci. Eng. A, 2003, vol. 363, pp. 171-178.
Dolan G. P, Flynn. R. J, Tanner. D. A, and Robinson. J. S: Mater. Sci. Technol., 2005, vol. 21(6), pp. 687-692.
Kavalco. P. M, Canale. L.C: J. ASTM Int., 2009, vol. 6, pp.1-20.
Dae-Hoon Ko, Dae-Cheol Ko, and Byung-Min Kim: Metall. Mater. Trans. B, 2015, vol. 46, pp. 2072-2083.
Shuhui Ma, Maniruzzaman, M. D. MacKenzie, D. S, and Sisson. R. D: Metall. Mater. Trans. B, 2007, vol. 38, pp. 583-589.
Tiryakioğlu Murat, and Ralph T. Shuey: Metall. Mater. Trans. B, 2007, vol. 38, pp. 575-582.
Starink. M. J, Milkereit. B, Zhang. Y, and Rometsch. P. A: Materials & Design 2015, vol. 88, pp. 958-971.
Dongfeng Li, Yin. B, Lei. Y, Liu. S, Deng. Y. L, and Zhang. X. M: Met. Mater. Int., 2016, vol. 22, pp. 222-228.
Liu. S. D, Chen. B, Li. C. B, Dai. Y, Deng. Y. L, and Zhang. X. M: Corros. Sci., 2015, vol. 91, pp. 203-212.
Tanner. D. A, and Robinson. J. S: J. Mater. Process. Technol., 2004, vol. 153, pp. 998-1004.
O.K. Abubakre, U.P. Mamaki, R.A. Muriana: J. Miner. Mater. Charact. Eng., 2009, vol. 8, pp. 303–15.
Chen. S. Y, Chen. K. H, Peng. G. S, Liang. X, and Chen. X. H: Transactions of Nonferrous Metals Society of China, 2012, vol. 22, pp. 47-52.
Zhang. L, Feng. X, Li. Z, and Liu. C: Proceedings of the Institution of Mechanical Engineers, Part B: Journal of Engineering Manufacture, 2013, vol. 227, pp. 954-964.
Le Masson P, Loulou T, Artioukhine E, Rogeon P, Carron D, and Quemener J. J: Int. J. Therm. Sci., 2002, vol. 41, pp. 517-527.
Li. Y. N, Zhang. Y. A, Li. X. W, Li. Z. H, Wang. G. J, Yan. H. W, Jin. L. B, Xiong. B. Q: Materials Science Forum, 2017, vol. 877, pp. 647-654.
B. Liscic, H.M. Tensi, L.C. Canale, G.E. Totten: Quenching theory and technology, CRC Press, Boca Raton, 2010, pp. 606-655.
Su. J, and Hewitt. G. F: Numer. Heat Transfer, Part A, 2004, vol. 45, pp. 777-789.
ASTM G110, Standard Practice for Evaluating Intergranular Corrosion Resistance of Heat Treatable Aluminum Alloys by Immersion in Sodium Chloride + Hydrogen Peroxide Solution, 2015.
A. E. Hughes, A. Boag, A. M. Glenn, D. McCulloch, T. H. Muster, C. Ryan, C. Luo, and X. Zhou: Corros. Sci., 2011, vol. 53, pp. 27-39.
A. Boag, A. E. Hughes, A. M. Glenn, T. H. Muster, and D. McCulloch: Corros. Sci., 2011, vol. 53, pp. 17-26.
E. McCafferty: Corros. Sci., 2005, vol. 47, pp. 3202–15.
Bergant. Z, Trdan. U, and Grum. J: Corros. Sci., 2014, vol. 88, pp. 372-386.
Heakal. F. E. T, Tantawy. N. S, and Shehta. O. S: Mater. Chem. Phys., 2011, vol. 130, pp. 743-749.
Zhang. X, Guo. M, Zhang. J, and Zhuang. L: Metall. Mater. Trans. B, 2016, vol. 47, pp. 608-620.
Zhao. Y. L, Yang. Z. Q, Zhang. Z, Su. G. Y, and Ma. X. L: Acta Mater., 2013, vol. 61, pp. 1624-1638.
Wang. S. C, and Starink. M. J: Int. Mater. Rev., 2005, vol. 50, pp. 193-215.
Wang. S. C, Starink. M. J, and Gao. N: Scr. Mater., 2006, vol. 54, pp. 287-291.
T. Ramgopal, P. I. Gouma, and G. S. Frankel: Corrosion, 2002, vol. 58, pp. 687-697.
Hashimoto. T, Zhang. X, Zhou. X, Skeldon. P, Haigh. S. J, and Thompson. G. E: Corros. Sci., 2016, vol. 103, pp. 157-164.
Birbilis. N, Cavanaugh. M. K, Kovarik. L, and Buchheit. R. G: Electrochem. Commun., 2008, vol. 10, pp. 32-37.
Wang. J, Zhang. B, Wu. B, and Ma. X. L: Corros. Sci., 2016, vol. 105, pp. 183-189.
Acknowledgments
This study was financially supported by the National Defense Supporting Research Program (JPPT-125-GJGG-08-01), and the experimental material was provided by Southwest Aluminum Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted October 16, 2017.
Rights and permissions
About this article
Cite this article
Yin, Y., Luo, B., Jing, H. et al. Influences of Quench Cooling Rate on Microstructure and Corrosion Resistance of Al-Cu-Mg Alloy Based on the End-Quenching Test. Metall Mater Trans B 49, 2241–2251 (2018). https://doi.org/10.1007/s11663-018-1329-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-018-1329-1