Abstract
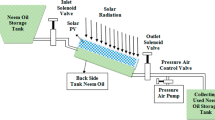
Cyclohexane dehydrogenation in the solar-driven membrane reactor is a promising method of directly producing pure hydrogen and benzene from cyclohexane and storing low-grade solar energy as high-grade chemical energy. In this paper, partial pressure of gases, conversion rate of cyclohexane, and energy efficiency of the reactor are analyzed based on numerical simulation. The process of cyclohexane dehydrogenation under four temperatures (200°C, 250°C, 300°C, and 350°C) and four permeate pressures (0.050 MPa, 0.025 MPa, 0.010 MPa, and 0.001 MPa) were studied. A complete conversion rate (99.9%) of cyclohexane was obtained as the reaction equilibrium shifts forward with hydrogen separation. The first-law thermodynamic efficiency, solar-to-fuel efficiency, and exergy efficiency could reach as high as 94.69%, 46.93% and 93.08%, respectively. This study indicates that it is feasible to combine solar energy supply technology with cyclohexane dehydrogenation reaction integrated with membrane reactor.
Similar content being viewed by others
Abbreviations
- C p :
-
specific heat capacity/kJ·(mol·K)−1
- d M :
-
membrane thickness/m
- Ex :
-
exergy/kJ·mol−1
- ΔG :
-
Gibbs free energy/J·mol−1
- HHV:
-
molar higher heating value/kJ·mol−1
- ΔH :
-
enthalpy change/kJ·mol−1
- J :
-
hydrogen permeation flux/mol/(m2·s)−1
- K p :
-
reaction equilibrium constant/Pa3
- P :
-
pressure/MPa
- P 0 :
-
atmosphere pressure/MPa
- Q rg :
-
solar thermal energy input for raising reactant temperature/kJ
- Q th :
-
enthalpy change of cyclohexane dehydrogenation/kJ
- Q sh :
-
thermal energy out of reactor/kJ
- R :
-
universal gas constant/J·(mol·K)−1
- r c :
-
kinetic reaction rate/mol·s−1
- ΔS :
-
entropy of reactions/J·(mol·K)−1
- T 0 :
-
room temperature/K
- T h :
-
reaction temperature/K
- T sun :
-
temperature of the sun surface/K
- ηabs :
-
absorption efficiency
- ηex :
-
exergy efficiency
- ηHHV :
-
first-law thermodynamic efficiency with separation exergy
- ηHHV,real :
-
first-law thermodynamic efficiency with real separation energy
- ηopt :
-
optical efficiency
- ηp :
-
vacuum pump efficiency
- ηs→e :
-
photoelectric efficiency
- ηs→f :
-
solar-to-fuel efficiency with separation exergy
- ηs→f,real :
-
solar-to-fuel efficiency with real separation energy
- ⊖:
-
standard state
- in:
-
input, inside
- init:
-
initial
- out:
-
output, outside
- p:
-
pump
- rg:
-
reactant gas
- res:
-
residual gas
References
Wang H.S., Solar thermochemical fuel generation. Wind Solar Hybrid Renewable Energy System, 2020. DOI:https://doi.org/10.5772/intechopen.90767.
Lewis N.S., Nocera D.G., Powering the planet: Chemical challenges in solar energy utilization. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(43): 15729–15735.
Guo S.P., Liu Q.B., Sun J., Jin H.G., A review on the utilization of hybrid renewable energy. Renewable and Sustainable Energy Reviews, 2018, 91: 1121–1147.
Kong H., Hao Y., Wang H.S., A solar thermochemical fuel production system integrated with fossil fuel heat recuperation. Applied Thermal Engineering, 2016, 108: 958–966.
Koutsonikolasa D., Kaldisb S., Zaspalisa V.T., Sakellaropoulos G.P., Potential application of a microporous silica membrane reactor for cyclohexane dehydrogenation. International Journal of Hydrogen Energy, 2012, 37(21): 16302–16307.
Ashfaq A.B., Samamtha B.C., Displacement of the benzene solvent molecule from Cr(CO)5(benzene) by piperidine: a laser flash photolysis experiment. Journal of Chemical Education, 2000, 77(10): 1348–1351.
Gao X.X., Gu Q., Ma J.Q., Zeng Y.F., MVR heat pump distillation coupled with ORC process for separating a benzene-toluene mixture. Energy, 2018, 143: 658–665.
Kumar S., Gaba T., Simulation of catalytic dehydrogenation of cyclohexane in zeolite membrane reactor. International Journal of Chemical Reactor Engineering, 2009, 7(1): 1–36.
Xia Z.J., Lu H.F., Liu H.Y., Zhang Z.K., Chen Y.F., Cyclohexane dehydrogenation over Ni-Cu/SiO2 catalyst: Effect of copper addition. Catalysis Communications, 2017, 90: 39–42.
Xia Z.J., Liu H.Y., Lu H.F., Zhang Z.K., Chen Y.F., Study on catalytic properties and carbon deposition of Ni-Cu/SBA-15 for cyclohexane dehydrogenation. Applied Surface Science, 2017, 422: 905–912.
Villaluenga J.P.G., Tabe-Mohammadi A., A review on the separation of benzene/cyclohexane mixtures by pervaporation processes. Journal of Membrane Science, 2020, 169(2): 159–174.
Itoh N., A Membrane Reactor Using Palladium. AIChE Journal, 1987, 33(9): 1576–1578.
DeLancey G.B., Kovenklioglu S., Ritter A.B., Schneider J.C., Cyclohexane dehydrogenation for thermochemical energy conversion. Industrial & Engineering Chemistry Process Design and Development, 1983, 22(4): 639–645.
Farsi M., Jahanmiri A., Mathematical simulation and optimization of methanol dehydration and cyclohexane dehydrogenation in a thermally coupled dual-membrane reactor. International Journal of Hydrogen Energy, 2011, 36 (22): 14416–14427.
Itoh N., A membrane reactor using palladium. American Institute of Chemical Engineers Journals, 1987, 33(9): 1576–1578.
Jeong B.H., Sotowa K.I., Kusakabe K., Catalytic dehydrogenation of cyclohexane in an FAU-type zeolite membrane reactor. Journal of Membrane Science, 2003, 224 (1–2): 151–158.
Hatlevik Ø., Gade S.K., Keeling M.K., Thoen P.M., Davidson A.P., Way J.D., Palladium and palladium alloy membranes for hydrogen separation and production: History, fabrication strategies, and current performance. Separation and Purification Technology, 2010, 73(1): 59–64.
Wang H.S., Liu M.K., Kong H., Hao Y., Thermodynamic analysis on mid/low temperature solar methane steam reforming with hydrogen permeation membrane reactors. Apply Thermal Engineering, 2018, 152: 925–936.
Wang B.Z., Kong H., Wang H.S., Wang Y.P., Hu X.J., Kinetic and thermodynamic analyses of mid/low-temperature ammonia decomposition in solar-driven hydrogen permeation membrane reactor. International Journal of Hydrogen Energy, 2019, 44(49): 26874–26887.
Uemiya S., Matsuda T., Kikuchi E., Hydrogen permeable palladium-silver alloy membrane supported on porous ceramics. Journal of Membrane Science, 1991, 56(3): 315–325.
Collins J.P., Way J.D., Preparation and characterization of a composite palladium-ceramic membrane. Industrial & Engineering Chemistry Research, 1993, 32(12): 3006–3013.
Morrealeb B.D., Cioccob M.V., Enickc R.M., Morsic B.I., Howarda B.H., Cuginia A.V., Rothenbergera K.S., The permeability of hydrogen in bulk palladium at elevated temperatures and pressures. Journal of Membrane Science, 2003, 212(1–2): 87–97.
Young J.R., Purity of hydrogen permeating through Pd, Pd-25% Ag and Ni. Review of Scientific Instruments, 1963, 34 (8): 891–892.
Schwartz S.E., Does fossil fuel combustion lead to global warming? Energy, 1993, 18(12): 1229–1248.
Uemiya S., Matsuda T., Kikuchi E., Hydrogen permeable palladium-silver alloy membrane supported on porous ceramics. Journal of Membrane Science, 1991, 56(3): 315–325.
Xu L., Sun F.H., Ma L.R., Li X.L., Yuan G.F., Lei D.Q., Zhu H.B., Zhang Q.Q., Xu E.S., Wang Z.F., Analysis of the influence of heat loss factors on the overall performance of utility-scale parabolic trough solar collectors. Energy, 2018, 162: 1077–1091.
Tou M., Michalsky R., Steinfeld A., Solar-driven thermochemical splitting of CO2 and in situ separation of CO and O2 across a ceria redox membrane reactor. Joule, 2017, 1(1): 146–154.
Wang H.S., Hao Y., Kong H., Thermodynamic study on solar thermochemical fuel production with oxygen permeation membrane reactors. International Journal of Energy Research, 2015, 39(13): 1790–1799.
Kong H., Kong X.H., Wang H.S., Wang J., A strategy for optimizing efficiencies of solar thermochemical fuel production based on nonstoichiometric oxides. International Journal of Hydrogen Energy, 2019, 44(36): 19585–19594.
Price H., Lüpfert E., Kearney D., Zarza E., Cohen G., Gee R., Mahoney R., Advances in parabolic trough solar power technology. Journal of Solar Energy Engineering, 2002, 124(2): 109–125.
Geyer M., Lüpfert E., Osuna R., Esteban A., Schiel W., Schweitzer A., Zarza E., Nava P., Langenkamp J., Mandelberg E., EUROTROUGH–Parabolic trough collector developed for cost efficient solar power generation. 11th International Symposium on Concentrating Solar Power and Chemical Energy Technologies, Zurich, Switzerland, 2002, 9: 4–6.
Wang H.S., Wang B.Z., Kong H., Lu X.F., Hu X.J., Thermodynamic analysis of methylcyclohexane dehydrogenation and solar energy storage via solar-driven hydrogen permeation membrane reactor. Membranes, 2020, 10(12): 374.
Sun J., Liu Q., Hong H., Numerical study of parabolic-trough direct steam generation loop in recirculation mode: Characteristics, performance and general operation strategy. Energy Conversion and Management, 2015, 96: 287–302.
Ishida M., Kawamura K., Energy and exergy analysis of a chemical process system with distributed parameters based on the enthalpy-direction factor diagram. Industrial & Engineering Chemistry Process Design and Development, 1982, 21(4): 690–695.
Bai Z., Liu Q.B., Gong L., Lei J., Application of a mid-/low-temperature solar thermochemical technology in the distributed energy system with cooling, heating and power production. Applied Energy, 2019, 253: 113491.
Wang H.S., Li W.J., Liu T., Liu X., Hu X.J., Thermodynamic analysis and optimization of photovoltaic/thermal hybrid hydrogen generation system based on complementary combination of photovoltaic cells and proton exchange membrane electrolyzer. Energy Conversion and Management, 2019, 183: 97–108.
Li W.J., Wang H.S., Hao Y., A PVTC system integrating photon-enhanced thermionic emission and methane reforming for efficient solar power generation. Science Bulletin, 2017, 62(20): 1380–1387.
Morreale B.D., Ciocco M.V., Enick R.M., Morsi B.I., Howard B.H., Cugini A.V., Rothenberger K.S., The permeability of hydrogen in bulk palladium at elevated temperatures and pressures. Journal of Membrane Science, 2003, 212(1–2): 87–97.
HSC chemistry 5.11, 2001–2002, Outokumpu Research Oy, Pori, Finland, A. Roine.
Wang H.S., Hao Y., Thermodynamic study of solar thermochemical methane steam reforming with alternating H2 and CO2 permeation membranes reactors. Energy Procedia, 2017, 105: 1980–1985.
Wang H.S., Wang B.Z, Qi X.Y., Wang J., Yang R.F., Li D.X., Hu X.J., Innovative non-oxidative methane dehydroaromatization via solar membrane reactor. Energy, 2021, 216: 119265.
Bulfin B., Call F., Lange M., Lóbben O., Sattler C., Pitz-Paal R., Shvets I.V., Thermodynamics of CeO2〉 thermochemical fuel production. Energy Fuel, 2015, 29 (2): 1001–1009.
Wang H.S., Kong H., Pu Z.G., Li Y., Hu X.J., Feasibility of high efficient solar hydrogen generation system integrating photovoltaic cell/photon-enhanced thermionic emission and high-temperature electrolysis cell. Energy Conversion and Management, 2020, 210: 112699.
Li W.J., Jin J., Wang H.S., Wei X., Ling Y.Y., Hao Y., Gang P., Jin H.G., Full-spectrum solar energy utilization integrating spectral splitting, photovoltaics and methane reforming. Energy Conversion and Management, 2018, 173: 602–612.
Li W.J., Hao Y., Wang H.S., Liu H., Sui J., Efficient and low–carbon heat and power cogeneration with photovoltaics and thermochemical storage. Applied Energy, 2017, 206: 1523–1531.
Acknowledgment
This work is funded by the National Natural Science Foundation of China (No. 51906179), the China Scholarship Council (No. 201906275035), and the National Key Research and Development Program of China (No. 2018YFC0808401).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, X., Wang, B., Wang, M. et al. Cyclohexane Dehydrogenation in Solar-Driven Hydrogen Permeation Membrane Reactor for Efficient Solar Energy Conversion and Storage. J. Therm. Sci. 30, 1548–1558 (2021). https://doi.org/10.1007/s11630-021-1392-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11630-021-1392-9