Abstract
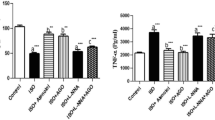
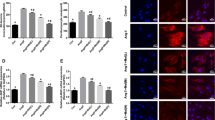
Melatonin is thought to have the ability of antiatherogenic, antioxidant, and vasodilatory. It is not only a promising protective in acute myocardial infarction but is also a useful tool in the treatment of pathological remodeling. However, its role in myocardial hypertrophy remains unclear. In this study, we investigated the protective effects of melatonin on myocardial hypertrophy induced by lipopolysaccharide (LPS) and to identify their precise mechanisms. The cultured myocardial cell was divided into six groups: control group, LPS group, LPS + ethanol (4%), LPS + melatonin (1.5 mg/ml) group, LPS + melatonin (3 mg/ml) group, and LPS + melatonin (6 mg/ml) group. The morphologic change of myocardial cell was observed by inverted phase contrast microscope. The protein level of myocardial cell was measured by Coomassie brilliant blue protein kit. The secretion level of tumor necrosis factor-α (TNF-α) was evaluated by enzyme-linked immunosorbent assay (ELISA). Ca2+ transient in Fura-2/AM-loaded cells was measured by Till image system. The expression of Ca2+/calmodulin-dependent kinase II (CaMKII) and calcineurin (CaN) was measured by Western blot analysis. Our data demonstrated that LPS induced myocardial hypertrophy, promoted the secretion levels of TNF-α, and increased Ca2+ transient level and the expression of CaMKII and CaN. Administration of melatonin 30 min prior to LPS stimulation dose-dependently attenuated myocardial hypertrophy. In conclusion, the results revealed that melatonin had the potential to protect against myocardial hypertrophy induced by LPS in vitro through downregulation of the TNF-α expression and retains the intracellular Ca2+ homeostasis.





Similar content being viewed by others
References
Anderson ME, Brown JH, Bers DM (2011) CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol 51:468–473
Arangino S, Cagnacci A, Angiolucci M, Vacca AM, Longu G, Volpe A, Melis GB (1999) Effects of melatonin on vascular reactivity, catecholamine levels, and blood pressure in healthy men. Am J Cardiol 83:1417–1419
Arras M, Hoche A, Bohle R, Eckert P, Riedel W, Schaper J (1996) Tumor necrosis factor-alpha in macrophages of heart, liver, kidney, and in the pituitary gland. Cell Tissue Res 285:39–49
Beltrami CA, Finato N, Rocco M, Feruglio GA, Puricelli C, Cigola E, Quaini F, Sonnenblick EH, Olivetti G, Anversa P (1994) Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circ 89:151–163
Berenji K, Drazner MH, Rothermel BA, Hill JA (2005) Does load-induced ventricular hypertrophy progress to systolic heart failure? Am J Physiol Heart Circ Physiol 289:H8–H16
Bogoyevitch MA, Andersson MB, Gillespie-Brown J, Clerk A, Glennon PE, Fuller SJ, Sugden PH (1996) Adrenergic receptor stimulation of the mitogen-activated protein kinase cascade and cardiac hypertrophy. Biochem J 314(Pt 1):115–121
Bozkurt B, Kribbs SB, Clubb FJ Jr, Michael LH, Didenko VV, Hornsby PJ, Seta Y, Oral H, Spinale FG, Mann DL (1998) Pathophysiologically relevant concentrations of tumor necrosis factor-alpha promote progressive left ventricular dysfunction and remodeling in rats. Circ 97:1382–1391
Chen CF, Wang D, Reiter RJ, Yeh DY (2011) Oral melatonin attenuates lung inflammation and airway hyperreactivity induced by inhalation of aerosolized pancreatic fluid in rats. J Pineal Res 50:46–53
Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F (1999) Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem 274:10689–10692
Cuzzocrea S, Costantino G, Mazzon E, Caputi AP (1999) Regulation of prostaglandin production in carrageenan-induced pleurisy by melatonin. J Pineal Res 27:9–14
Diwan A, Tran T, Misra A, Mann DL (2003) Inflammatory mediators and the failing heart: a translational approach. Curr Mol Med 3:161–182
Dominguez-Rodriguez A, Abreu-Gonzalez P, Reiter RJ (2012) Melatonin and cardioprotection in the acute myocardial infarction: a promising cardioprotective agent. Int J Cardiol 158:309–310
Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O’Donnell SE, Aykin-Burns N et al (2008) A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 133:462–474
Frey N, Katus HA, Olson EN, Hill JA (2004) Hypertrophy of the heart: a new therapeutic target? Circ 109:1580–1589
Fu M, Zhang J, Xu S, Pang Y, Liu N, Tang C (2001) Role of calcineurin in angiotensin II-induced cardiac myocyte hypertrophy of rats. Chin Med Sci J 16:1–4
Galano A, Tan DX, Reiter RJ (2011) Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res 51:1–16
Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450
Gunther S, Grossman W (1979) Determinants of ventricular function in pressure-overload hypertrophy in man. Circ 59:679–688
Hardeland R, Tan DX, Reiter RJ (2009) Kynuramines, metabolites of melatonin and other indoles: the resurrection of an almost forgotten class of biogenic amines. J Pineal Res 47:109–126
Heymans S, Hirsch E, Anker SD, Aukrust P, Balligand JL, Cohen-Tervaert JW, Drexler H, Filippatos G, Felix SB, Gullestad L et al (2009) Inflammation as a therapeutic target in heart failure? A scientific statement from the translational research committee of the heart failure association of the european society of cardiology. Eur J Heart Fail 11:119–129
Korkmaz A, Reiter RJ, Topal T, Manchester LC, Oter S, Tan DX (2009) Melatonin: an established antioxidant worthy of use in clinical trials. Mol Med 15:43–50
Liu CJ, Cheng YC, Lee KW, Hsu HH, Chu CH, Tsai FJ, Tsai CH, Chu CY, Liu JY, Kuo WW et al (2008) Lipopolysaccharide induces cellular hypertrophy through calcineurin/NFAT-3 signaling pathway in H9c2 myocardiac cells. Mol Cell Biochem 313:167–178
Lu M, Wang H, Wang J, Zhang J, Yang J, Liang L, Maslov LN (2013) Astragaloside IV protects against cardiac hypertrophy via inhibiting the Ca2+/CaN signaling pathway. Planta Med 80(1):63–69. doi:10.1055/s-0033-1360129
Manda K, Ueno M, Anzai K (2007) AFMK, a melatonin metabolite, attenuates X-ray-induced oxidative damage to DNA, proteins and lipids in mice. J Pineal Res 42:386–393
Menendez-Pelaez A, Reiter RJ (1993) Distribution of melatonin in mammalian tissues: the relative importance of nuclear versus cytosolic localization. J Pineal Res 15:59–69
Nakamura K, Fushimi K, Kouchi H, Mihara K, Miyazaki M, Ohe T, Namba M (1998) Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-alpha and angiotensin II. Circ 98:794–799
Oliveira LG, Kuehn CC, dos Santos CD, Miranda MA, da Costa CM, Mendonca VJ, do Prado Junior JC (2013) Protective actions of melatonin against heart damage during chronic Chagas disease. Acta Trop 128:652–658
Ortiz F, Garcia JA, Acuna-Castroviejo D, Doerrier C, Lopez A, Venegas C, Volt H, Luna-Sanchez M, Lopez LC, Escames G (2014) The beneficial effects of melatonin against heart mitochondrial impairment during sepsis: inhibition of iNOS and preservation of nNOS. J Pineal Res 56:71–81
Perez MJ, Castano B, Gonzalez-Buitrago JM, Marin JJ (2007) Multiple protective effects of melatonin against maternal cholestasis-induced oxidative stress and apoptosis in the rat fetal liver-placenta-maternal liver trio. J Pineal Res 43:130–139
Reiter RJ, Tan DX (2003) What constitutes a physiological concentration of melatonin? J Pineal Res 34:79–80
Reiter RJ, Melchiorri D, Sewerynek E, Poeggeler B, Barlow-Walden L, Chuang J, Ortiz GG, Acuna-Castroviejo D (1995) A review of the evidence supporting melatonin’s role as an antioxidant. J Pineal Res 18:1–11
Reiter RJ, Tan DX, Manchester LC, Pilar Terron M, Flores LJ, Koppisepi S (2007) Medical implications of melatonin: receptor-mediated and receptor-independent actions. Adv Med Sci 52:11–28
Reiter RJ, Manchester LC, Fuentes-Broto L, Tan D-X (2010) Cardiac hypertrophy and remodelling: pathophysiological consequences and protective effects of melatonin. J Hypertens 28:S7–S12
Saito T, Fukuzawa J, Osaki J, Sakuragi H, Yao N, Haneda T, Fujino T, Wakamiya N, Kikuchi K, Hasebe N (2003) Roles of calcineurin and calcium/calmodulin-dependent protein kinase II in pressure overload-induced cardiac hypertrophy. J Mol Cell Cardiol 35:1153–1160
Sekiguchi K, Li X, Coker M, Flesch M, Barger PM, Sivasubramanian N, Mann DL (2004) Cross-regulation between the renin-angiotensin system and inflammatory mediators in cardiac hypertrophy and failure. Cardiovasc Res 63:433–442
Shioi T, Matsumori A, Kihara Y, Inoko M, Ono K, Iwanaga Y, Yamada T, Iwasaki A, Matsushima K, Sasayama S (1997) Increased expression of interleukin-1 beta and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in the hypertrophied and failing heart with pressure overload. Circ Res 81:664–671
Simko F, Paulis L (2013) Antifibrotic effect of melatonin—perspective protection in hypertensive heart disease. Int J Cardiol 168:2876–2877
Simko F, Pechanova O, Pelouch V, Krajcirovicova K, Celec P, Palffy R, Bednarova K, Vrankova S, Adamcova M, Paulis L (2010) Continuous light and L-NAME-induced left ventricular remodelling: different protection with melatonin and captopril. J Hypertens 28(Suppl 1):S13–S18
Simpson P (1985) Stimulation of hypertrophy of cultured neonatal rat heart cells through an alpha 1-adrenergic receptor and induction of beating through an alpha 1- and beta 1-adrenergic receptor interaction. Evidence for independent regulation of growth and beating. Circ Res 56:884–894
Singh MV, Kapoun A, Higgins L, Kutschke W, Thurman JM, Zhang R, Singh M, Yang J, Guan X, Lowe JS et al (2009) Ca2+/calmodulin-dependent kinase II triggers cell membrane injury by inducing complement factor B gene expression in the mouse heart. J Clin Invest 119:986–996
Singh MV, Swaminathan PD, Luczak ED, Kutschke W, Weiss RM, Anderson ME (2012) MyD88 mediated inflammatory signaling leads to CaMKII oxidation, cardiac hypertrophy and death after myocardial infarction. J Mol Cell Cardiol 52:1135–1144
Wang GJ, Wang HX, Yao YS, Guo LY, Liu P et al (2012) The role of Ca2+/calmodulin-dependent protein kinase II and calcineurin in TNF-alpha-induced myocardial hypertrophy. Braz J Med Biol Res 45:1045–1051
Yang RB, Mark MR, Gray A, Huang A, Xie MH, Zhang M, Goddard A, Wood WI, Gurney AL, Godowski PJ (1998) Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nat 395:284–288
Yokoyama T, Nakano M, Bednarczyk JL, McIntyre BW, Entman M, Mann DL (1997) Tumor necrosis factor-alpha provokes a hypertrophic growth response in adult cardiac myocytes. Circ 95:1247–1252
Zhang H, Liu D, Wang X, Chen X, Long Y, Chai W, Zhou X, Rui X, Zhang Q, Wang H et al (2013) Melatonin improved rat cardiac mitochondria and survival rate in septic heart injury. J Pineal Res 55:1–6
Zhu W, Zou Y, Shiojima I, Kudoh S, Aikawa R, Hayashi D, Mizukami M, Toko H, Shibasaki F, Yazaki Y et al (2000) Ca2+/calmodulin-dependent kinase II and calcineurin play critical roles in endothelin-1-induced cardiomyocyte hypertrophy. J Biol Chem 275:15239–15245
Acknowledgment
This study was supported by grants from the Innovation and demonstration of Nantong Social Science (S11954).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: T. Okamoto
Qi Lu and Xin Yi contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure 1
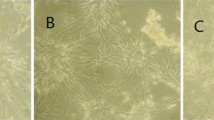
The volume of myocardial cell in each group. Photomicrographs of myocardial cell cultured in each group were taken by inverted phase contrast microscope and analyzed for size of cells by CIAS Daheng image analysis system. The shape of myocardial cell was fusiform, irregular triangle or polygon (A). The volume of myocardial cell in TNF-α group and TNF-α+ethanol group was obviously augmented compared with control group. The volume of myocardial cell in TNF-α+melatonin group was smaller than that of TNF-αgroup and TNF-α+ethanol group and was similar to that of control group (A, B). ∆ p< 0.01, vs. control group; * p< 0.01, vs. TNF-αgroup; # p< 0.01, vs. TNF-α+ethanol group. (GIF 29 kb)
Figure 2
The protein level of myocardial cell in each group. The protein level of myocardial cell was measured by coomassie brilliant blue protein kit. The total protein level of myocardial cell in TNF-αgroup and TNF-α+ethanol group was more than that of control group and was was similar to that of control group. ∆ p< 0.01, vs. control group; * p< 0.01, vs. TNF-αgroup; # p< 0.01, vs. TNF-α+ethanol group. (GIF 10 kb)
Rights and permissions
About this article
Cite this article
Lu, Q., Yi, X., Cheng, X. et al. Melatonin protects against myocardial hypertrophy induced by lipopolysaccharide. In Vitro Cell.Dev.Biol.-Animal 51, 353–360 (2015). https://doi.org/10.1007/s11626-014-9844-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-014-9844-0