Abstract
Background
Systematic screening skin examination has been proposed to reduce melanoma-related mortality.
Objective
To assess the potential effectiveness of screening, in a demographic at high risk of melanoma mortality.
Design
A cohort Markov state-transition model was developed comparing systematic screening versus usual care (no systematic screening). In the base case, we evaluated a sensitivity and specificity of 20% and 85%, respectively, for usual care (incidental detection) and 50% sensitivity and 85% specificity from systematic screening. We examined a wide range of values in sensitivity analyses.
Participants
Potential screening strategies applied to a hypothetical population of 10,000 white men from ages 50–75.
Main Measures
Incremental cost-effectiveness ratio, measured in cost per quality adjusted life year (QALY).
Key Results
Using base case assumptions, screening every 2 years beginning at age 60 reduced melanoma mortality by 20% with a cost-utility of $26,503 per QALY gained. Screening every 2 years beginning at age 50 reduced mortality by 30% with an incremental cost-utility of $67,970 per QALY. Results were sensitive to differences in accuracy of systematic screening versus usual care, and costs of screening, but were generally insensitive to costs of biopsy or treatment.
Conclusions
Assuming moderate differences in accuracy with systematic screening versus usual care, screening for melanoma every 2 years starting at age 50 or 60 may be cost-effective in white men. Results are sensitive to degree of difference in sensitivity with screening compared to usual care. Better studies of the accuracy of systematic screening exams compared with usual care are required to determine whether a trial of screening should be undertaken.
Similar content being viewed by others
INTRODUCTION
Approximately 10,000 Americans die annually from melanoma.1 According to Surveillance, Epidemiology, and End Result (SEER) data from the National Cancer Institute (NCI), the incidence of invasive melanoma has increased dramatically from 8 per 100,000 in 1975 to 25 per 100,000 in 2014.2 Given that mortality has also increased over the same time period, efforts have been made to encourage population-based screening by clinicians, including primary care physicians, to improve early detection.3 In 2016, the US Preventive Services Task Force (USPSTF) concluded that there was insufficient evidence to assess the balance of benefits and harms of visual skin examination by a clinician to screen for melanoma in adults.4 However, the Agency for Healthcare Research and Quality (AHRQ) suggested future research focus on assessing the effectiveness of targeted screening higher risk patient populations.5
While melanoma incidence has risen across demographic sub-groups, increases in mortality have been largely concentrated among white men over 65 years old, for whom mortality has increased from 9 to 24 per 100,000 between 1975 and 2014.2 In contrast, melanoma mortality among white women over 65 years old has increased from 6 to 9 per 100,000 between 1975 and 2014. Given this disproportionate burden of melanoma-specific mortality among older white men, targeting this easily identifiable high-risk sub-group for screening may be a reasonable intervention strategy. For that reason, we sought to use modeling to study cost-effectiveness of melanoma screening in white men.
While a randomized trial is theoretically attractive for determining the effectiveness of periodic skin examinations, such a trial would require very large sample sizes to adequately power comparisons, and many years of follow-up, making it quite expensive.6 In the absence of large randomized controlled trials comparing screening strategies, clinical decision analysis and cost-effectiveness or cost-utility modeling can be used to explicitly compare alternative clinical options. Previous cost-effectiveness analyses of melanoma screening have suggested that screening could potentially be cost-effective in certain high-risk groups such as those with a family history of melanoma.7 These studies, however, did not focus on the importance of the sensitivity and specificity of the screening visual skin examination. We define “systematic screening” as skin examination in the absence of skin-related complaints by a primary care provider (PCP), compared with “usual care,” defined as incidental diagnosis by a PCP or self-detection by a patient leading to a clinic visit.
In this analysis, we developed a Markov model to evaluate the cost-effectiveness of a set of skin cancer systematic screening strategies compared with usual care (no systematic screening) in a hypothetical population of 10,000 white men aged 50–75. Furthermore, we varied the sensitivity and specificity of the visual skin examination compared to usual care in order to evaluate the impact on the cost-effectiveness of different screening strategies.
METHODS
Overview and Model Structure
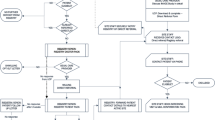
We developed a cohort state-transition Markov model to evaluate several screening strategies of different starting ages and intervals for screening, compared to usual care (Figure S1). A hypothetical population of 10,000 white men begins in the healthy state and then may transition every 12 months. We used annual cycles as they are commonly used and mimic annual visits for routine PCP visits. We modeled “undetected” and “detected” melanoma in situ, localized disease, regional disease, and metastatic disease as separate health states; costs, quality of life, and mortality rates differ in each state.
Our primary outcome measure was the incremental cost-effectiveness ratio (ICER), measured in cost per quality adjusted life year (QALY). We assumed a willingness to pay (WTP) threshold of ≤ $50,000 per QALY in the base case, but we also examined other thresholds within the cost-effectiveness acceptability curves (CEAC). Total costs (in 2018 US dollars) and QALYs were calculated (discounting at 3%/year for costs and utilities) over a lifetime horizon using a US health care system payer perspective, as recommended by the Second US Panel on Cost-Effectiveness.8 We also report clinical outcomes including melanoma mortality, incidence of melanoma by stage, and total cost of screening for each screening strategy. Total cost of screening was the sum of the cost of screening exams and biopsies for each strategy, less the sum of cost of biopsies in the base case (no screening) strategy.
Melanoma Incidence and Rates of Progression
We used age-adjusted melanoma incidence data from 2008 to 2012 SEER Medicare data for Caucasian men.8 We calibrated the transition probabilities for melanoma progression in the base model (no screening, i.e., usual care) to approximate data on age-adjusted melanoma mortality rate from 2008 to 2012 SEER Medicare data for Caucasian men, life expectancy after melanoma diagnosis, and melanoma stage distribution at diagnosis.9 More details of the model calibration are included in Table S2.
Screening Strategies
The median age of melanoma diagnosis is 63 years, and the median age at death is 69 years. Given that nearly 70% of melanomas are diagnosed after 55, we began our screening strategies at age 50 with intervals of 1, 2, or 5 years.9 We also modeled identical intervals beginning at age 60. Screening continued until patients reached age 75.
Test Characteristics for Usual Care vs. Skin Cancer Screening: Sensitivity and Specificity
Because of the limited data on the accuracy of screening or usual detection, we modeled a wide range of test performance characteristics. In the base case “no screening” arm, we assumed a sensitivity and specificity of 20% and 85%, respectively, for detection of any stage of melanoma, based on usual care and incidental detection; we assumed a sensitivity and specificity of 50% and 85%, respectively, for systematic screening examinations.10,11,12 The sensitivity of usual care or screening determined the transition probabilities between the “undetected” and “detected” health states (Figure S1). The specificity determined the number of false positive screens, which we accounted for in the model by incurring cost of screening and biopsy while remaining in the “healthy” state.
Cost and Utility Inputs
Table 1 contains the base case estimates and ranges for our key parameters. Screening costs were determined by the average total allowed amount for a level 3 evaluation and management examination of a return Medicare patient.13 We assumed screening and biopsy costs of $73 and $105, respectively.13 Costs for treatment were determined based upon previously published literature, which report approximate costs by melanoma stage, as well as expert opinion.14, 15 Cost estimates for stage 3 and 4 melanoma relied heavily on expert opinion, given that published data are not readily available in the era of immunotherapy and targeted therapy for melanoma. We therefore varied costs widely. We chose health state utilities based on previous estimates and expert opinion.14, 16 We assumed a decrement in quality of life after diagnosis of melanoma in situ because of the potential for anxiety related to diagnosis of cancer.17
Deterministic and Probabilistic Sensitivity Analysis
We conducted one- and two-way sensitivity analyses to examine the influence of key parameters. In the one-way analysis, we examined the effect of varying costs and health state utilities on preferred screening strategy. We used a two-way analysis to show how preferred screening strategy changes when we varied sensitivity of usual care as well as sensitivity of the screening visual skin examination.
To represent the collective uncertainty in our analyses, we conducted a global probabilistic sensitivity analysis (PSA) using second-order Monte Carlo simulation (n = 1000 trials) to determine the effect of parameter uncertainty on the probability of cost-effectiveness (Crystal Ball, Oracle). The parameter ranges used in the PSA are reported in Table 1. We used gamma distributions to parameterize costs and beta distributions to parameterize health state utilities.18 We did not vary transition probabilities probabilistically given that we calibrated these parameters to observed melanoma mortality rate and stage distribution at diagnosis. Finally, we show CEACs to illustrate how the probability of cost-effectiveness changes with willingness-to-pay threshold.19 This study was exempt from IRB.
RESULTS
Base Case
Our main results are shown in Table 2. Screening every year starting at age 50 had the fewest deaths from melanoma. However, this screening strategy was also associated with the highest number of detected MIS cases, the highest total cost of skin cancer screening, and the highest total healthcare costs per individual.
Annual screening starting at age 50 was associated with the highest total QALYs (per individual) over a lifetime horizon, followed by annual screening starting at age 60. “No screening” had the lowest QALYs and lowest costs. Assuming a WTP of $50,000 per QALY gained and our base case estimates of sensitivity of usual care and screening, the preferred screening strategy was screening every 2 years starting at age 60, with an ICER of $26,503 per QALY gained (Table 3). Screening every 2 years starting at age 50 had an incremental cost per QALY of $67,970 and would be the preferred strategy using a WTP threshold of $100,000 per QALY gained. Annual screening at 50 had an ICER of $116,640 and would be preferred strategy at higher WTP thresholds.
Deterministic Sensitivity Analysis
When we varied individual parameters throughout the ranges listed in Table 1, we found that the preferred screening strategy was sensitive to costs of screening and metastatic melanoma treatment, but not sensitive to costs of individual screening exams, biopsy, melanoma treatment, nor to variation in health state utilities (Table S1). Notably, screening was no longer preferred at a screening cost greater than $146, which was a 2-fold increase in cost over our base case assumption of $73 per screening exam. When the cost for metastatic melanoma treatment was increased to $750,000 to account for the introduction of new immunotherapy treatments, 20 we found that annual screening starting at age 60 was the preferred strategy.
Because of the importance of the differential sensitivity of usual care and screening, we performed two-way sensitivity analyses by varying the sensitivity of usual care (10–60%) and the sensitivity of screening (30–90%). We found that the ICERs and preferred screening strategies varied significantly with changes in the sensitivity of usual care vs. sensitivity of screening (Table 4).
Table 4 shows how the ICERs changed for our preferred screening strategy at WTP threshold of $50,000/QALY gained of every 2 years starting at age 60. For screening every 2 years starting at age 60, we found that the ICER remained under $50,000/QALY gained for two scenarios: (1) sensitivity of usual care = 10%, sensitivity of screening ranging from 30 to 70%; and (2) sensitivity of usual care = 20%, sensitivity of screening ranging from 50 to 90%.
Assuming a WTP of $50,000/QALY gained, screening every 2 years starting at age 60 was the preferred strategy only for the base case scenario (Table 4). When sensitivity of screening improves, or sensitivity of usual care gets worse, the preferred strategy is to screen every 2 years starting at age 50. Furthermore, for screening to be cost-effective compared with usual care, we see that the difference in sensitivity of usual care vs. screening must be at approximately + 30 percentage points or more, at least for cases where usual care is less than 40% (Table 4).
Probabilistic Sensitivity Analysis
At a WTP threshold of $50,000 per QALY gained, the preferred screening strategy is to screen every 2 years starting at 60, with an ICER of $26,664 (95% CI $18,135–$36,390) and a probability of cost-effectiveness of 94%. However, both the preferred strategy and probability of cost-effectiveness vary dramatically by WTP threshold (Fig. 1). Using a WTP threshold of $25,000 per QALY gained, no screening was the preferred strategy in 61% of simulations, with screening every 2 years starting at age 60 being the preferred strategy in 38% of simulations. At a WTP threshold of $100,000 per QALY gained, the preferred strategy was to screen every 2 years, starting at age 50. However, this was the preferred strategy in only 52% of simulations; screening every year starting at age 50 was the preferred strategy in 32% of the simulations.
The CEACs change dramatically under different assumptions for sensitivity of usual care vs. screening. When both usual care and systematic screening have relatively high sensitivity for detecting melanoma (usual care 60%; screening 90%), we found that no screening is the preferred strategy for the entire range of tested WTP thresholds (CEAC not shown). However, under more favorable screening assumptions, no screening was preferred (Figure S2).
DISCUSSION
We found that screening every 2 years has the potential to reduce melanoma mortality by 20 to 30% in older white men aged 50–75 under reasonable assumptions about the accuracy of systematic screening examinations by clinicians compared with usual care. Given a willingness to pay of $50,000 per quality adjusted life year gained, screening should begin at age 60; if society is willing to pay $100,000 per life year gained, screening should begin at age 50. Cost-effectiveness was minimally dependent upon input costs of screening or melanoma treatment costs, but strongly dependent upon the assumptions made about the sensitivity of systematic screening compared with usual care, defined as no systematic screening (incidental detection).
Our results suggest that accurately quantifying the sensitivity of screening examinations compared with usual care is an important factor in determining the effectiveness and cost-effectiveness of screening. We consider “usual care” as melanoma incidentally detected by patients or by physicians during routine clinical exam, in the absence of systematic screening. Importantly, the higher the sensitivity of usual care, the less cost-effective systematic screening. Unfortunately, there are no high-quality studies comparing the sensitivity of screening examination to usual care. One fair quality cohort study performed in Queensland, Australia, assessed the effectiveness of skin cancer screening of clinicians and reported a 40.2% sensitivity and 86.1% specificity at 36 months of follow-up.11 However, this study did not measure usual care: clinicians were trained and encouraged to perform skin examinations to detect skin cancer.21 A separate Australian cohort study evaluated the effectiveness of melanoma screening by dermatologists or plastic surgeons and reported a sensitivity of 69.7% at 12 months, declining to 49% at 24 months of follow-up. Specificity in this study was 97.6%.12 While this study did measure sensitivity and specificity of screening among dermatologists and plastic surgeons, it did not measure the sensitivity and specificity of usual care. Importantly, while there are many studies of diagnostic accuracy which report sensitivity for the diagnosis of melanoma ranging between 42 to 100% among primary care clinician (PCP) and 81 to 100% among dermatologists, most of these studies involve the evaluation of images of individual pigmented lesions, which does not represent true screening or usual care.10 In practice few patients have full body skin exams, less than one third of primary care clinicians report screening their patients for skin cancer, and in a national survey of white middle-aged and older white men, only 15.7% reported having a skin exam in the past year.22, 23
Previous cost-effective modeling studies have attempted to approximate the effects of screening versus no screening.24,25,26 One modeling study of adults above 20 years old estimated that a one-time skin cancer screening would cost $29,170 per year of life saved compared to no screening. In men over 50, the cost was $15,580.26 The model was sensitive to the cost of screening, melanoma prevalence, and the probability of detecting localized disease; however, investigators did not vary the sensitivity or specificity of the screening examination, rather they assumed the difference in screen versus no screen as the difference in distribution between an America Academy of Dermatology screening program and SEER stage distributions.26, 27 In an Australian study modeling screening for melanoma by PCPs, investigators varied the sensitivity of visual screening and found that at a sensitivity of 60%, screening every 2 years for 20 years was cost-effective at $25,000 per life year saved for men aged 50 years and $43,000 per life year saved for women aged 50 years.25 However, this study did not adequately define the parameters of their usual care estimates. Lack of detailed evaluation of usual care is shortcoming of previous work modeling skin cancer screening strategies. Our study addresses this shortcoming by varying the sensitivity of usual care against the sensitivity of screening, which is a critical in the decision of whether or not to implement a screening program.
Our modeling has several strengths. Given the uncertainty about accuracy, we focused on the importance of sensitivity of systematic screening compared with usual care. Furthermore, in this analysis, we updated costs from previous studies and conducted multiple sensitivity analyses, including probabilistic analysis.24 As with previous studies, we found that melanoma screening could be cost-effective in a high-risk group at a willingness to pay of $50,000 per QALY.24,25,26
There are other risks associated with skin cancer screening that were not integrated into the model. Screening has potential associated harms including biopsy scarring, skin infections, and risk of overdiagnosis and overtreatment of cancers that may not be destined to progress. Overdiagnosis is of particular concern in melanoma in which incidence has quadrupled in the United States, and mortality across the population has not increased at a commensurate rate.28, 29 As part of skin cancer screening, it is also likely that incidental non-lethal, non-melanoma skin cancers (which are more common than melanoma) such as basal cell carcinoma and squamous cell carcinoma will be detected, resulting in an reduced morbidity, but an increase in cost.30
Limitations
Our modeling study has limitations. Although the natural history data is based on the most current resources, modeling the behavior of melanoma and its relationship with screening involves uncertainty. Some melanomas may have a long radial growth phase, making them amenable to discovering during screening. However, some melanomas may have a rapid vertical growth phase causing them to quickly invade local tissues, making them less amenable to the benefits of screening. We attempted to mitigate this shortcoming by calibrating model transition probabilities to approximate age-adjusted melanoma mortality rate from SEER Medicare data for white men as well as published reports of stage distribution for melanoma at diagnosis. We limited the population in this study to middle-aged and older white men because we wanted to test the best case for screening in a high-risk group. Our findings may not be applicable to other groups such as younger men, women, and non-whites. Further modeling work will examine the cost-effectiveness in lower risk groups, such as women and non-white men.
CONCLUSION
Screening for melanoma every 2 years starting at age 50 or 60 may be cost-effective in white men. Results are sensitive to the degree of difference in sensitivity with screening compared to usual care. Future studies should consider a more accurate determination of the sensitivity and specificity of systematic screening for melanoma as compared to usual care.
References
Chen ST, Geller AC, Tsao H. Update on the Epidemiology of Melanoma. Curr Dermatol Rep. 2013;2(1):24-34.
SEER*Stat Database (1975-2016). Bethesda, MD: National Cancer Institute Surveillance Research Program, 2019 (http://www.seer.cancer.gov).
Ferris LK, Saul MI, Lin Y, et al. A Large Skin Cancer Screening Quality Initiative: Description and First-Year Outcomes. JAMA Oncol. 2017;3(8):1112-1115.
Force USPST. Screening for skin cancer: Us preventive services task force recommendation statement. JAMA. 2016;316(4):429-435.
Wernli KJ, Henrikson NB, Morrison CC, Nguyen M, Pocobelli G, Blasi PR. Screening for Skin Cancer in Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2016;316(4):436-447.
Geller AC, Swetter SM, Oliveria S, Dusza S, Halpern AC. Reducing mortality in individuals at high risk for advanced melanoma through education and screening. J Am Acad Dermatol. 2011;65(5 Suppl 1):S87-94.
Gordon LG, Rowell D. Health system costs of skin cancer and cost-effectiveness of skin cancer prevention and screening: a systematic review. Eur J Cancer Prev. 2015;24(2):141-149.
Sanders GD, Neumann PJ, Basu A, et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA. 2016;316(10):1093-1103.
Howlader NNA, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review. In. Bethesda, MD1975-2013.
Chen SC, Bravata DM, Weil E, Olkin I. A comparison of dermatologists' and primary care physicians' accuracy in diagnosing melanoma: a systematic review. Arch Dermatol. 2001;137(12):1627-1634.
Aitken JF, Janda M, Elwood M, Youl PH, Ring IT, Lowe JB. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006;54(1):105-114.
Fritschi L, Dye SA, Katris P. Validity of melanoma diagnosis in a community-based screening program. Am J Epidemiol. 2006;164(4):385-390.
Medicare Provider Utilization and Payment Data: Physician and Other Supplier https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Provider-Charge-Data/Physician-and-Other-Supplier.html. Accessed February 6th, 2017.
Styperek A, Kimball AB. Malignant melanoma: The implications of cost for stakeholder innovation. Am J Pharm Ben. 2012;4(2):66-76.
Guy GP, Jr., Ekwueme DU, Tangka FK, Richardson LC. Melanoma treatment costs: a systematic review of the literature, 1990-2011. Am J Prev Med. 2012;43(5):537-545.
King SM, Bonaccorsi P, Bendeck S, et al. Melanoma quality of life: pilot study using utility measurements. Arch Dermatol. 2011;147(3):353-354.
Livingstone E, Krajewski C, Eigentler TK, et al. Prospective evaluation of follow-up in melanoma patients in Germany - results of a multicentre and longitudinal study. Eur J Cancer. 2015;51(5):653-667.
Briggs AH, Weinstein MC, Fenwick EA, et al. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-6. Med Decis Making. 2012;32(5):722-732.
Barton GR, Briggs AH, Fenwick EA. Optimal cost-effectiveness decisions: the role of the cost-effectiveness acceptability curve (CEAC), the cost-effectiveness acceptability frontier (CEAF), and the expected value of perfection information (EVPI). Value Health. 2008;11(5):886-897.
Oh A, Tran DM, McDowell LC, et al. Cost-Effectiveness of Nivolumab-Ipilimumab Combination Therapy Compared with Monotherapy for First-Line Treatment of Metastatic Melanoma in the United States. J Manag Care Spec Pharm. 2017;23(6):653-664.
Aitken JF, Elwood JM, Lowe JB, Firman DW, Balanda KP, Ring IT. A randomised trial of population screening for melanoma. J Med Screen. 2002;9(1):33-37.
Kirsner RS, Muhkerjee S, Federman DG. Skin cancer screening in primary care: prevalence and barriers. J Am Acad Dermatol. 1999;41(4):564-566.
Coups EJ, Geller AC, Weinstock MA, Heckman CJ, Manne SL. Prevalence and correlates of skin cancer screening among middle-aged and older white adults in the United States. Am J Med. 2010;123(5):439-445.
Losina E, Walensky RP, Geller A, et al. Visual screening for malignant melanoma: a cost-effectiveness analysis. Arch Dermatol. 2007;143(1):21-28.
Girgis A, Clarke P, Burton RC, Sanson-Fisher RW. Screening for melanoma by primary health care physicians: a cost-effectiveness analysis. J Med Screen. 1996;3(1):47-53.
Freedberg KA, Geller AC, Miller DR, Lew RA, Koh HK. Screening for malignant melanoma: A cost-effectiveness analysis. J Am Acad Dermatol. 1999;41(5 Pt 1):738-745.
Koh HK, Norton LA, Geller AC, et al. Evaluation of the American Academy of Dermatology's National Skin Cancer Early Detection and Screening Program. J Am Acad Dermatol. 1996;34(6):971-978.
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30.
Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605-613.
Waldmann A, Nolte S, Geller AC, et al. Frequency of excisions and yields of malignant skin tumors in a population-based screening intervention of 360,288 whole-body examinations. Arch Dermatol. 2012;148(8):903-910.
Contributors
Study concept and design: Adamson, Pignone
Acquisition, analysis, and interpretation of data: All Authors
Drafting of the manuscript: Adamson, Jarmul
Critical revision of the manuscript for important intellectual content: All Authors
Statistical analysis: Jarmul
Obtained funding: Adamson
Administrative, technical, or material support: Adamson
Study supervision: Pignone
IRB Statement
This study was exempt from IRB.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Pignone is a former member of the US Preventive Services Task Force. The views expressed here are his and not necessarily those of the Task Force.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 665 kb)
Rights and permissions
About this article
Cite this article
Adamson, A.S., Jarmul, J.A. & Pignone, M.P. Screening for Melanoma in Men: a Cost-Effectiveness Analysis. J GEN INTERN MED 35, 1175–1181 (2020). https://doi.org/10.1007/s11606-019-05443-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-019-05443-3