Abstract
Importance
Intensive lifestyle change (e.g., the Diabetes Prevention Program) and metformin reduce type 2 diabetes risk among patients with prediabetes. However, real-world uptake remains low. Shared decision-making (SDM) may increase awareness and help patients select and follow through with informed options for diabetes prevention that are aligned with their preferences.
Objective
To test the effectiveness of a prediabetes SDM intervention.
Design
Cluster randomized controlled trial.
Setting
Twenty primary care clinics within a large regional health system.
Participants
Overweight/obese adults with prediabetes (BMI ≥ 24 kg/m2 and HbA1c 5.7–6.4%) were enrolled from 10 SDM intervention clinics. Propensity score matching was used to identify control patients from 10 usual care clinics.
Intervention
Intervention clinic patients were invited to participate in a face-to-face SDM visit with a pharmacist who used a decision aid (DA) to describe prediabetes and four possible options for diabetes prevention: DPP, DPP ± metformin, metformin only, or usual care.
Main Outcomes and Measures
Primary endpoint was uptake of DPP (≥ 9 sessions), metformin, or both strategies at 4 months. Secondary endpoint was weight change (lbs.) at 12 months.
Results
Uptake of DPP and/or metformin was higher among SDM participants (n = 351) than controls receiving usual care (n = 1028; 38% vs. 2%, p < .001). At 12-month follow-up, adjusted weight loss (lbs.) was greater among SDM participants than controls (− 5.3 vs. − 0.2, p < .001).
Limitations
Absence of DPP supplier participation data for matched patients in usual care clinics.
Conclusions and Relevance
A prediabetes SDM intervention led by pharmacists increased patient engagement in evidence-based options for diabetes prevention and was associated with significantly greater uptake of DPP and/or metformin at 4 months and weight loss at 12 months. Prediabetes SDM may be a promising approach to enhance prevention efforts among patients at increased risk.
Trial Registration
This study was registered at clinicaltrails.gov (NCT02384109)).
Similar content being viewed by others
INTRODUCTION
Shared decision-making is a hallmark of patient-centered care and supports patients to achieve informed decisions aligned with their preferences.1, 2 Shared decision-making (SDM) incorporating decision aids (DA) reduce decisional conflict and improves patient knowledge, risk perceptions, and satisfaction with care.3, 4 SDM was included in the 2001 Institute of Medicine5 report and Section 3506 of the Affordable Care Act (ACA).6 SDM has been applied in many clinical conditions7,8,9 but we are not aware of any prior applications for diabetes prevention.
Prediabetes is an ideal SDM scenario since it is a preference-sensitive condition with several effective prevention strategies (intensive lifestyle intervention, such as the Diabetes Prevention Program [DPP], metformin, or both).10,11,12,13 Intensive lifestyle intervention has the greatest overall diabetes risk reduction, but care guidelines also endorse metformin for higher risk patients (individuals who are obese, < 60 years of age, and women with a history of gestational diabetes).11,12,13,14,15,16,17,18 Although there is no FDA indication for use in prediabetes, metformin has been shown to be safe, well tolerated, and potentially cost saving.11,12,13,14,15,16,17,18 SDM can help align patient’s choices regarding diabetes prevention with their personal preferences for care. Since only 11% of the estimated 84 million US adults with prediabetes are aware of their diagnosis,19 SDM using a high-quality DA should increase patient awareness of prediabetes, which in turn may be associated with greater adoption of diabetes prevention strategies.20
We tested the effectiveness of a prediabetes SDM intervention among overweight/obese patients with prediabetes on several important outcomes, including uptake of intensive lifestyle change (≥ 9 sessions DPP and/or metformin) and weight loss at 12-month follow-up. We hypothesized that a prediabetes SDM intervention would be associated with greater uptake of DPP and/or metformin and greater weight loss as compared with usual care. To our knowledge, this is the first study to assess the effectiveness of a SDM intervention for patients with prediabetes.
METHODS
Design Overview
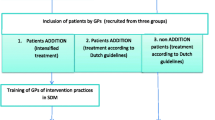
We designed a cluster randomized trial, with clinics as the unit of randomization, to examine (1) uptake of DPP and/or metformin and (2) weight change among overweight/obese adults with prediabetes who participated in SDM for diabetes prevention versus propensity score–matched patients receiving usual care. The study was approved by the Institutional Review Board at the University of California, Los Angeles.
Setting and Participants
We conducted this study from 2015 to 2018 at UCLA Health, which includes an extensive primary care network in the greater Los Angeles region. We stratified 20 primary care clinics by clinic size and mean patient age, randomizing 10 clinics to the SDM intervention and 10 to usual care (we launched in 16 clinics [8 intervention and 8 control] and subsequently added the last 4). We used electronic medical record (EMR) data to identify overweight/obese patients (body mass index [BMI] ≥ 24 kg/m2 or ≥ 22 kg/m2 if Asian) with prediabetes (HbA1c 5.7–6.4% within the prior 3 months) between 18 and 74 years of age. We excluded patients with diabetes (any HbA1c > 6.4% or International Classification of Diseases [ICD] 250.xx/diabetes problem list or antiglycemic medications and/or insulin), chronic kidney disease (estimated glomerular filtration index ≤ 45 ml/min), active eating disorder(s), and women who had polycystic ovary syndrome or were pregnant or planning to get pregnant in the next year.
After identifying potentially eligible patients, we notified their primary care providers (PCPs) who had the option of excluding patients for whom they felt the study was inappropriate (e.g., terminal illness, inability to tolerate 150 weekly minutes of physical activity). PCPs could also refer other patients with prediabetes to the study. All eligible patients received a standardized invitation letter signed by their PCP to schedule a visit with a pharmacist to learn about prediabetes and their options for diabetes prevention.
Intervention
The SDM intervention was delivered by clinical pharmacists already working collaboratively with PCPs in each clinic. We provided training in SDM and DA use, with quarterly refresher training sessions throughout the trial. Healthwise, a national provider of health information and patient decision support tools for over 35 years, produced the DA. The DA entitled, “Prediabetes: Which Treatment Should I Use?”, provides information about prediabetes, intensive lifestyle change, and metformin as two evidence-based options for diabetes prevention, and summarizes the relative risk reduction as well as potential side effects of each option. The DA meets quality standards established by the International Participant Decision Aid Standards (IPDAS) collaboration.21
Patients met face-to-face with an SDM pharmacist in a private room in their usual primary care clinic. After confirming patient eligibility and documenting written informed consent, the pharmacist delivered the SDM intervention over 35–45 min. The DA was presented in the form of a web-based interactive tool on the computer available in the clinic room. The pharmacist and patient went through the standardized DA material together, which was presented in six sequential steps: (1) Get the Facts, (2) Compare Options, (3) Your Feelings, (4) Your Decision, (5) Quiz Yourself, and (6) Your Summary. The DA introduced the concept of having choices for diabetes prevention and described four possible prevention options—(1) DPP alone, (2) metformin alone, (3) DPP together with metformin, or (4) usual primary care. Pharmacists walked patients through the DA steps and helped them explore their preferences and make decisions. Patients were provided a printed copy of a summary report with their decision and plan at the end of the SDM visit. Pharmacists also completed an EMR note template to communicate patient’s choices with PCPs. Patients choosing metformin received a prescription from the pharmacist after PCPs indicated approval. Pharmacists ordered follow-up labs to monitor renal function as needed.22 Patients choosing DPP were referred to local DPP providers participating in the CDC Diabetes Prevention Recognition Program (DPRP). The intervention did not cover costs for DPP or metformin prescriptions.
Outcomes of Interest
Our primary outcome was DPP (≥ 9 sessions attended) and/or metformin uptake at 4-month follow-up (data up to 8 months post-SDM was included). We received DPP attendance data for intervention patients from DPP suppliers. Because it was not feasible to collect informed consent from matched controls, DPP suppliers could not share DPP participation data from controls. Therefore, we conducted natural language queries of all EMR progress notes between 2015 and 2018 to capture participation in DPP or any other structured weight loss program. Metformin uptake was assessed using EMR medication reconciliation notes.
A clinically important secondary outcome was weight change at 12 months. We used EMR weight data. Baseline weights for SDM participants were within 2 weeks (before or after) the SDM visit. We used the closest available weight measures within 9–16 months to define the 12-month outcome.
Statistical Analyses
As this was the first prediabetes SDM trial, we did not have prior data to estimate effect sizes. Given the low rates of uptake of intensive lifestyle change (< 5%20) or metformin use (3.7%23), a differential increase of 12% points of either strategy among intervention patients as compared with controls seemed clinically meaningful (since this represents about a twofold increase from national estimates). Using an intraclass correlation coefficient (ICC) of 0.007 (from a primary care cluster randomized trial of sedentary patients intending to start an exercise program), enrolling 560 patients provided 90% power to detect a difference between arms in our primary outcome. We conservatively estimated 25% attrition at 12 months and planned to recruit 700 participants (350 SDM patients and 350 patients from usual care clinics) to maintain 90% power for the primary outcome.
We used propensity score matching to define a control group. We modeled a propensity score among all eligible, contacted patients in SDM clinics to predict the likelihood of SDM participation (enrollment). The model coefficients were then used to derive propensity scores on which to match for all eligible patients in control clinics. To control for secular time-trends, we identified controls with HbA1c results within 3 months of our intervention patients. We conducted a 3:1 match with replacement using the following individual-level variables: age, gender, race, ethnicity, income, BMI, hemoglobin A1c, eGFR, insurance type, number of comorbidities (0, 1, 2, 3+), and frequency of baseline provider visits. We also included 11 comorbidity indicators for hypertension, hyperlipidemia, coronary artery disease, chronic obstructive pulmonary disease, atrial fibrillation/arrhythmia, stroke, peripheral vascular disease, osteoarthritis, depression, anxiety, and substance abuse.
We used generalized linear mixed effects models to compare (1) DPP and/or metformin uptake at 4 months and (2) weight change at 12 months between groups. Our baseline date for intervention patients was their SDM visit day and controls were assigned the same baseline date to whom they were matched (i.e., pseudo-baseline). Weight change models used an intent-to-treat analytic approach and included fixed effects for time, treatment group and time-treatment interactions, and random effects of patients and clinics to account for repeated measurements within patients and clinic clustering. We also adjusted for the days from baseline weight to the start of the study window since this was different between arms.
To address 12-month missing weight data, we conducted a sensitivity analysis using pattern-mixture modeling24, 25 with control-based pattern imputation (generated 12-month weight change if a follow-up weight assessment was not available between 9 and 16 months). Since baseline gender and HbA1c were statistically different between groups, we conducted a second sensitivity analysis adjusting for these variables. We also adjusted for rates of weight loss medication use (intervention 4.6% vs. control 3.6%, p = 0.42) since we had not included this in the propensity score. Three additional sensitivity analyses examined interaction terms between intervention and individual-level variables to assess if effectiveness of the SDM intervention varied by age (< 60 vs. ≥ 60 years), BMI (< 35 vs. ≥ 35 kg/m2), or baseline HbA1c (5.7–5.9 vs. 6.0–6.4%). A sixth sensitivity analysis was conducted to remove weight change outliers (we excluded 5% of the sample, 2.5% from the bottom and 2.5% from the top).
We also calculated our ICC, representing the proportion of total variation in DPP and/or metformin uptake attributable to the clinic level, using a formula described by Wu et al.26 All analyses were done using SAS, version 9.3 (SAS Institute), and STATA, version 14.2 (StataCorp).
Role of the Funding Source
This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (R18 grant number DK105464). The funding source had no role in the study design, conduct, reporting, or decision to submit the manuscript for publication.
RESULTS
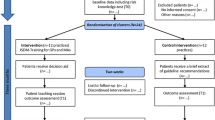
Between November 2015 and 2016, EMR screening and PCP referrals yielded 1555 potentially eligible participants from 10 SDM clinics; 1222 met all initial eligibility criteria. We excluded 64 patients based on PCP advice, leaving 1158 who were mailed SDM invitation letters. We were able to reach 680 patients and 52% (n = 351) of them completed the SDM intervention between November 2015 and October 2016 (Fig. 1).
Our propensity score matching identified 1046 control patients from 10 usual care clinics. After excluding anyone missing baseline weights or meeting other exclusion criteria (n = 18), we had 1028 control patients. As compared with controls, SDM participants were less likely to be female (58.7 vs. 66.8%, p = 0.006) and had a small difference in baseline HbA1c (5.96 vs. 5.94%, p = 0.033, Table 1). Time from baseline weight to the start of the assigned study window was significantly different between groups (1.4 days for intervention patients vs. 41 days for controls, p < .001).
Primary Outcome—Uptake of DPP and/or Metformin at 4-Month Follow-up
Over 83% of SDM participants selected a diabetes prevention strategy (DPP, metformin, or both); 260 chose DPP (with or without metformin) and 32% (n = 83) completed ≥ 9 DPP sessions. In comparison, 0.4% of control patients (n = 7) had any EMR evidence of lifestyle change participation (DPP or any other weight loss program). SDM participants were also more likely to use metformin than control patients (19% vs. 1.6%, p < .001). Overall, uptake of DPP and/or metformin was 38% among SDM participants vs. 2% in control patients at 4-month follow-up (p < .001) (Table 2).
Secondary Outcome—Weight Change at 12-Month Follow-up
Weight documentation at 12 months was available for 89% (n = 312) of SDM participants and 83% (n = 856) of usual care patients between 9 and 16 months. Unadjusted mean weight loss at 12-month follow-up was higher among SDM than usual care participants (− 5.2 lbs. [SD 11.2] vs. − 0.2 lbs. [SD 10.9] (p < .001)) (Table 3). The adjusted difference in mean weight loss between groups was − 5.1 lbs. (CI − 6.5, − 3.7, p < .001). Mean percent weight loss was also 2.7% higher among SDM than usual care participants (95% CI − 3.4, − 2.0, p < .001).
Sensitivity analyses to address missing 12-month follow-up weight assessments (11% for intervention patients and 17% for controls), adjust for baseline differences in gender and HbA1c and use of weight loss medications, and remove extreme outliers did not significantly change results (see Table 4). Models incorporating interaction terms between intervention and age (< 60, ≥ 60 years), BMI (< 35, ≥ 35 kg/m2), or baseline HbA1c (5.7–5.9, 6.0–6.4%) also did not significantly change results. Our ICC for uptake of a diabetes prevention strategy was 0.026, indicating that less than 3% of the variation in uptake was explained by differences among SDM and usual control clinics.
DISCUSSION
We found a prediabetes SDM intervention delivered by pharmacists in primary care led to significantly higher DPP and/or metformin uptake at 4 months and greater weight loss at 12 months as compared with usual care. To our knowledge, the PRIDE study is one of the first studies to use SDM for prediabetes and to translate both evidence-based arms of the DPP (lifestyle, metformin) in real-world primary care.
Over one-third of SDM participants had DPP (≥ 9 sessions) and/or metformin uptake as compared with 2% of control patients. Although intensive lifestyle change and metformin are both effective options for diabetes risk reduction, uptake of both strategies remains very low in real-world settings. National estimates of any intensive lifestyle change uptake among patients with prediabetes are not readily available but participant-level evaluation of the National CDC DPRP included data on 14,747 US adults enrolled in the National DPP between 2012 and 2016 in an estimated pool of over 80 million Americans with prediabetes.27 In addition, a systematic review of DPP implementation in real-world settings found that 71% (n = 25) of studies achieved low participation rates, half of which were participation rates ≤ 10%.28 Similarly, studies have shown that metformin is rarely used for individuals with prediabetes, with prior estimates ranging between < 1 and 8.1%.29 The low levels of DPP and/or metformin uptake in real-world settings provide needed context to assess the magnitude of effects observed in this trial. The fact that 38% patients who completed a one-time SDM visit, lasting between 35 and 45 min, engaged in DPP and/or used metformin is noteworthy.
The significant weight loss observed among intervention participants further confirms the effectiveness of prediabetes SDM. We assessed weight outcomes using objective EMR data and an intent-to-treat analytic approach for all participants (i.e., we included those who did not choose a diabetes prevention strategy during SDM and those that did not follow through with DPP and/or metformin). The overall adjusted difference in weight loss between groups was − 5.1 lbs. at 12 months. This degree of weight loss is clinically meaningful for overweight/obese patients with prediabetes since every kilogram of weight loss (2.2 lbs.) was associated with a 16% relative diabetes risk reduction over 3 years of follow-up in the DPP study.30 The fact that only 11% of adults with prediabetes are aware of their diagnosis19 underscores the importance of an SDM approach to help improve prediabetes awareness, willingness to consider options for diabetes prevention, and perhaps improved adherence to DPP and/or metformin.31
Recent studies have shown that individuals with prediabetes consider both intensive lifestyle interventions and metformin as reasonable options.32 However, studies have shown that up to three-fourths of adults with prediabetes are not provided with an appropriate plan during a PCP visit.33 Providers infrequently address prediabetes during visits and even when prediabetes is addressed, there is variability in the options presented to patients.34 To effectively address these gaps, prediabetes care must be enhanced without increasing the burden on already-taxed PCPs, who typically address numerous competing medical demands during time-limited visits. Since health systems are increasingly incorporating allied health professionals to work collaboratively with PCPs to manage chronic conditions,35,36,37,38,39,40,41,42,43,44,45,46 such models may also be a natural fit to address gaps in care for patients with prediabetes.
Overall, prediabetes is an ideal clinical condition to apply SDM since prevalence of this condition is high, awareness is low, and several reasonable and effective options are available to patients. One strategy to simultaneously help enhance prediabetes care delivery without over burdening PCPs is to include prediabetes SDM visits led by pharmacists or other allied health care professionals. Future studies should examine prediabetes SDM delivery led by other allied health care professionals since pharmacists may not be widely available in all health systems.
This study has several important limitations. First, this trial was conducted at UCLA Health where pharmacists were integrated in a large network of primary care clinics, which may limit generalizability. However, the UCLA Health system spans a very large region with a mix of primary care clinic settings and our sample included 38% AA and Hispanic participants. Second, the intervention patients who chose to participate may have been more motivated than others to lower their diabetes risk. To help address possible selection effects, we created a propensity score predicting the likelihood of study enrollment and used this to identify comparable (i.e., “control”) patients who would have a similar propensity to enroll. Finally, we did not receive data from DPP suppliers for controls. However, we used EMR queries to examine uptake of intensive lifestyle interventions among controls and conservatively included any indication of participation in any lifestyle change program as meeting the criteria for uptake.
CONCLUSIONS
We found that a pharmacist-led SDM intervention for diabetes prevention was associated with higher uptake of DPP and/or metformin at 4-month and weight loss at 12-month follow-up. Eighty-four million US adults have prediabetes but most are unaware of their diagnosis and few engage in evidence-based therapies, such as intensive lifestyle change and/or metformin, to reduce their risk of incident type 2 diabetes. Shared decision-making is critically important to increase prediabetes awareness and help patients make informed decisions regarding options for diabetes prevention that align with their preferences and values which they are willing to follow through on.
References
Elwyn G, Frosch D, Thomson R, Joseph-Williams N, Lloyd A, Kinnersley P, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361–7. doi:https://doi.org/10.1007/s11606-012-2077-6
Elwyn G, Laitner S, Coulter A, Walker E, Watson P, Thomson R. Implementing shared decision making in the NHS. BMJ. 2010;341:c5146. doi:https://doi.org/10.1136/bmj.c5146
Stacey D, Bennett CL, Barry MJ, Col NF, Eden KB, Holmes-Rovner M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011(10):CD001431. doi:https://doi.org/10.1002/14651858.CD001431.pub3
Mullan RJ, Montori VM, Shah ND, Christianson TJ, Bryant SC, Guyatt GH, et al. The diabetes mellitus medication choice decision aid: a randomized trial. Arch Intern Med. 2009;169(17):1560–8. doi:https://doi.org/10.1001/archinternmed.2009.293 [doi]
Institute of Medicine. 2001. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington: The National Academies Press. https://www.nap.edu/catalog/10027/crossing-the-quality-chasm-a-new-health-system-for-the Accessed May 15, 2019.
Oshima Lee E, Emanuel EJ. Shared decision making to improve care and reduce costs. N Engl J Med. 2013;368(1):6–8. doi:https://doi.org/10.1056/NEJMp1209500
Allen JD, Akinyemi IC, Reich A, Fleary S, Tendulkar S, Lamour N. African American Women’s Involvement in Promoting Informed Decision-Making for Prostate Cancer Screening Among Their Partners/Spouses. Am J Mens Health. 2018;12(4):884–93. doi:https://doi.org/10.1177/1557988317742257
Kane HL, Halpern MT, Squiers LB, Treiman KA, McCormack LA. Implementing and evaluating shared decision making in oncology practice. CA Cancer J Clin. 2014;64(6):377–88. doi:https://doi.org/10.3322/caac.21245
Ankuda CK, Block SD, Cooper Z, Correll DJ, Hepner DL, Lasic M, et al. Measuring critical deficits in shared decision making before elective surgery. Patient Educ Couns. 2014;94(3):328–33. doi:https://doi.org/10.1016/j.pec.2013.11.013
Kattan M, ed. Encyclopedia of medical decision making. SAGE Publications; 2009.
Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi:https://doi.org/10.1056/NEJMoa012512
Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677–86. doi:https://doi.org/10.1016/S0140-6736(09)61457-4
Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3(11):866–75. doi:https://doi.org/10.1016/S2213-8587(15)00291-0
American Diabetes Association. Standards of medical care in diabetes--2016. Diabetes Care. 2016;39 Suppl 1:S1–112.
Diabetes Prevention Program Research Group. HbA1c as a predictor of diabetes and as an outcome in the diabetes prevention program: a randomized clinical trial. Diabetes Care. 2015;38(1):51–8. doi:https://doi.org/10.2337/dc14-0886
Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35(4):731–7. doi:https://doi.org/10.2337/dc11-1299
Herman WH, Edelstein SL, Ratner RE, Montez MG, Ackermann RT, Orchard TJ, et al. Effectiveness and cost-effectiveness of diabetes prevention among adherent participants. Am J Manag Care. 2013;19(3):194–202.
Diabetes Prevention Program Research Group. The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent-to-treat analysis of the DPP/DPPOS. Diabetes Care. 2012;35(4):723–30. doi:https://doi.org/10.2337/dc11-1468
Centers for Disease Ccontrol and Prevention. Awareness of prediabetes--United States, 2005–2010. MMWR Morb Mortal Wkly Rep. 2013;62(11):209–12.
Gopalan A, Lorincz IS, Wirtalla C, Marcus SC, Long JA. Awareness of Prediabetes and Engagement in Diabetes Risk-Reducing Behaviors. Am J Prev Med. 2015;49(4):512–9. doi:https://doi.org/10.1016/j.amepre.2015.03.007
The Ottawa Hospital Research Institute. Patient Decision Aids: Decision Aid Summary. http://decisionaid.ohri.ca/Azsumm.php? ID=1654. Accessed May 15, 2019.
FDA. Drug Safety Communications. 2016. http://www.fda.gov/downloads/Drugs/DrugSafety/UCM494140.pdf. Accessed Accessed May 15, 2019.
Moin T, Li J, Duru OK, Ettner S, Turk N, Keckhafer A, et al. Metformin prescription for insured adults with prediabetes from 2010 to 2012: a retrospective cohort study. Ann Intern Med. 2015;162(8):542–8. doi:https://doi.org/10.7326/M14-1773
Little RJ, Wang Y. Pattern-mixture models for multivariate incomplete data with covariates. Biometrics. 1996;52(1):98–111.
Ratitch B, O’Kelly M, Tosiello R. Missing data in clinical trials: from clinical assumptions to statistical analysis using pattern mixture models. Pharm Stat. 2013;12(6):337–47. doi:https://doi.org/10.1002/pst.1549
Wu S, Crespi CM, Wong WK. Comparison of methods for estimating the intraclass correlation coefficient for binary responses in cancer prevention cluster randomized trials. Contemp Clin Trials. 2012;33(5):869–80. doi:https://doi.org/10.1016/j.cct.2012.05.004
Ely EK, Gruss SM, Luman ET, Gregg EW, Ali MK, Nhim K, Rolka DB, Albright AL. A National Effort to Prevent Type 2 Diabetes: Participant-Level Evaluation of CDC’s National Diabetes Prevention Program. Diabetes Care. 2017;4010.
Aziz Z, Absetz P, Oldroyd J, Pronk NP, Oldenburg B. A systematic review of real-world diabetes prevention programs: learnings from the last 15 years. Implement Sci :IS. 2015;10:172. doi:https://doi.org/10.1186/s13012-015-0354-6
Moin T, Schmittdiel JA, Flory JH, Yeh J, Karter AJ, Kruge LE, Schillinger D, Mangione CM, Herman WH, Walker EA. Review of Metformin Use for Type 2 Diabetes Prevention. Am J Prev Med. 2018 Oct;55(4):565–574. doi: https://doi.org/10.1016/j.amepre.2018.04.038.
Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29(9):2102–7. doi:https://doi.org/10.2337/dc06-0560
Bauer AM, Parker MM, Schillinger D, Katon W, Adler N, Adams AS, et al. Associations between antidepressant adherence and shared decision-making, patient-provider trust, and communication among adults with diabetes: diabetes study of Northern California (DISTANCE). J Gen Intern Med. 2014;29(8):1139–47. doi:https://doi.org/10.1007/s11606-014-2845-6
O’Brien MJ, Moran MR, Tang JW, Vargas MC, Talen M, Zimmermann LJ, et al. Patient Perceptions About Prediabetes and Preferences for Diabetes Prevention. Diabetes Educ. 2016;42(6):667–77. doi:https://doi.org/10.1177/0145721716666678
Mainous AG 3rd, Tanner RJ, Baker R. Prediabetes Diagnosis and Treatment in Primary Care. Journal of the American Board of Family Medicine. J Am Board Fam Med. 2016;29(2):283–5. doi:https://doi.org/10.3122/jabfm.2016.02.150252
Marshall C, Adams S, Dyer W, Schmittdiel J. Opportunities to Reduce Diabetes Risk in Women of Reproductive Age: Assessment and Treatment of Prediabetes within a Large Integrated Delivery System. Womens Health Issues. 2017;27(6):666–72. doi:https://doi.org/10.1016/j.whi.2017.06.001
Katon W, Von Korff M, Lin E, Simon G. Rethinking practitioner roles in chronic illness: the specialist, primary care physician, and the practice nurse. Gen Hosp Psychiatry. 2001;23(3):138–44.
Willard-Grace R, Chen EH, Hessler D, DeVore D, Prado C, Bodenheimer T, et al. Health coaching by medical assistants to improve control of diabetes, hypertension, and hyperlipidemia in low-income patients: a randomized controlled trial. Ann Fam Med. 2015;13(2):130–8. doi:https://doi.org/10.1370/afm.1768
Celeste-Harris S, Maryniuk M. Educating Medical Office Staff: Enhancing Diabetes Care in Primary Care Offices. Diabetes Spectr. 2006;19(2):84–9.
Cranor CW, Christensen DB. The Asheville Project: Short-term outcomes of a community pharmacy diabetes care program. J Am Pharm Assoc (2003). 2012;52(6):838–50. https://doi.org/10.1331/JAPhA.2012.12542
Smith M, Bates DW, Bodenheimer TS. Pharmacists belong in accountable care organizations and integrated care teams. Health Aff (Millwood). 2013;32(11):1963–70. https://doi.org/10.1377/hlthaff.2013.0542
Weber CA, Ernst ME, Sezate GS, Zheng S, Carter BL. Pharmacist-physician comanagement of hypertension and reduction in 24-hour ambulatory blood pressures. Arch Intern Med. 2010;170(18):1634–9. doi:https://doi.org/10.1001/archinternmed.2010.349
Hirsch JD, Steers N, Adler DS, Kuo GM, Morello CM, Lang M, et al. Primary care-based, pharmacist-physician collaborative medication-therapy management of hypertension: a randomized, pragmatic trial. Clin Ther. 2014;36(9):1244–54. doi:https://doi.org/10.1016/j.clinthera.2014.06.030
Ramalho de Oliveira D, Brummel AR, Miller DB. Medication therapy management: 10 years of experience in a large integrated health care system. J Manag Care Pharm. 2010;16(3):185–95. doi:https://doi.org/10.18553/jmcp.2010.16.3.185
Carter BL, Ardery G, Dawson JD, James PA, Bergus GR, Doucette WR, et al. Physician and pharmacist collaboration to improve blood pressure control. Arch Intern Med. 2009;169(21):1996–2002. doi:https://doi.org/10.1001/archinternmed.2009.358
Nelson K, Pitaro M, Tzellas A, Lum A. Practice profile. Transforming the role of medical assistants in chronic disease management. Health Aff (Millwood). 2010;29(5):963–5. doi:https://doi.org/10.1377/hlthaff.2010.0129
Massimi A, De Vito C, Brufola I, Corsaro A, Marzuillo C, Migliara G, et al. Are community-based nurse-led self-management support interventions effective in chronic patients? Results of a systematic review and meta-analysis. PloS One. 2017;12(3):e0173617. doi:https://doi.org/10.1371/journal.pone.0173617
Weeks G, George J, Maclure K, Stewart D. Non-medical prescribing versus medical prescribing for acute and chronic disease management in primary and secondary care. Cochrane Database Syst Rev. 2016;11:CD011227. doi:https://doi.org/10.1002/14651858.CD011227.pub2
Acknowledgments
The authors would like to thank Mr. RM for his help with project coordination, Mr. SL for help with data acquisition, and Ms. KS, Ms. SS, Dr. WS, and Ms. MK for their help with DPP implementation/delivery. The authors would also like to thank Drs. SLE and GM for their input throughout the trial. The authors would also like to thank Healthwise for providing the DA at no cost to the study and Dr. MB for facilitating the collaboration. The authors would also like to thank all the clinics, providers, and patients who made this study possible.
Funding
This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (R18DK105464). Dr. Moin also receives support from the Department of Veterans Affairs (QUE15-272, QUE15-286, and CSP2002). Dr. Mangione receives support from the University of California at Los Angeles (UCLA), Resource Centers for Minority Aging Research Center for Health Improvement of Minority Elderly under National Institutes of Health (NIH)/NIA Grant P30-AG021684, and from NIH/National Center for Advancing Translational Sciences UCLA Clinical and Translational Science Institute Grant UL1TR000124. Dr. Mangione holds the Barbara A. Levey and Gerald S. Levey Endowed Chair in Medicine, which partially supported her work. Drs. Duru’s effort is also supported in part by the University of California, Los Angeles, Resource Center for Minority Aging Research, Center for Health Improvement of Minority Elderly (RCMAR/CHIME) under NIH/NIA Grant P30-AG021684 and NIH Career Development Award K08-AG033360. Dr. Mangione is a member of the U.S. Preventive Services Task Force.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the Institutional Review Board at the University of California, Los Angeles. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest
Dr. Duru is on the Healthwise scientific board. None of the other authors disclosed any potential conflicts of interest.
Disclaimer
The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the views of the National Institutes of Health (NIH) and the US Preventive Services Task Force.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moin, T., Duru, O.K., Turk, N. et al. Effectiveness of Shared Decision-making for Diabetes Prevention: 12-Month Results from the Prediabetes Informed Decision and Education (PRIDE) Trial. J GEN INTERN MED 34, 2652–2659 (2019). https://doi.org/10.1007/s11606-019-05238-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-019-05238-6