Abstract
Background
Rectal bleeding is a common, frequently benign problem that can also be an early sign of colorectal cancer. Diagnostic evaluation for rectal bleeding is complex, and clinical practice may deviate from available guidelines.
Objective
To assess the degree to which primary care physicians document risk factors for colorectal cancer among patients with rectal bleeding and order colonoscopies when indicated, and the likelihood of physicians ordering and patients receiving recommended colonoscopies based on demographic characteristics, visit patterns, and clinical presentations.
Design
Cross-sectional study using explicit chart abstraction methods.
Participants
Three hundred adults, 40–80 years of age, presenting with rectal bleeding to 15 academically affiliated primary care practices between 2012 and 2016.
Main Measures
1) The frequency at which colorectal cancer risk factors were documented in patients’ charts, 2) the frequency at which physicians ordered colonoscopies and patients received them, and 3) the odds of ordering and patients receiving recommended colonoscopies based on patient demographic characteristics, visit patterns, and clinical presentations.
Key Results
Risk factors for colorectal cancer were documented between 9% and 66% of the time. Most patients (89%) with rectal bleeding needed a colonoscopy according to a clinical guideline. Physicians placed colonoscopy orders for 74% of these patients, and 56% completed the colonoscopy within a year (36% within 60 days). The odds of physicians ordering recommended colonoscopies were significantly higher in patients aged 50–64 years of age than in those aged 40–50 years (OR = 2.23, 95% CI: 1.04, 4.80), and for patients whose most recent colonoscopy was 5 or more years ago (OR = 4.04, 95% CI: 1.50, 10.83). The odds of physicians ordering and patients receiving recommended colonoscopies were significantly lower for each primary care visit unrelated to rectal bleeding (OR = 0.85, 95% CI: 0.75, 0.96).
Conclusions
Diagnostic evaluation of patients presenting to primary care with rectal bleeding may be suboptimal because of inadequate risk factor assessment and prioritization of patients’ other concurrent medical problems.
Similar content being viewed by others
INTRODUCTION
Colorectal cancer is the second leading cause of cancer-related deaths in the United States, and early detection can increase the likelihood of early-stage presentation and survival.1,2,–3 Rectal bleeding is a common experience affecting 14–24% of adults.4,5,–6 Although frequently benign,7 it can also be a sign of colorectal cancer.3,8 Improving the quality of the diagnostic evaluation for rectal bleeding may not only improve colorectal cancer detection, but could also reduce mortality.8,9
Available studies suggest that diagnostic errors are prevalent in the primary care setting and are associated with serious adverse patient outcomes (e.g., death).10,11,–12 The diagnostic evaluation of rectal bleeding can be complex because it involves collecting and appraising a number of risk factors for colorectal cancer, each with limited specificity or predictive value, including association with anemia, change in bowel habits, unintentional weight loss, and personal or family history of adenomas or colorectal cancer.8,13 Evaluations of rectal bleeding frequently culminate in a colonoscopy, which can also be a source of diagnostic error or delay if there is inadequate communication and coordination between providers in primary care and specialty care settings (e.g., failure to inform patient that follow-up tests are needed, scheduling delays, or lack of timely follow-up).14,15,16,17,18,–19 Additionally, certain patient populations may be at greater risk for diagnostic errors or delays. For example, investigators have previously found that patients who are female, non-white, with lower income, or who have Medicaid or Medicare insurance were less likely than their male, white, higher-income, or commercially insured counterparts to receive complete assessments when they presented to primary care with rectal bleeding.15
Several studies—most conducted nearly a decade ago (i.e., 2006–2010) have investigated the diagnostic evaluation of patients presenting to primary care with rectal bleeding.15,20,21,22,–23 Both academic primary care and Veterans Affairs (VA) settings have been studied, but for the non-VA setting, this timing is notable for several reasons. First, these studies were conducted prior to the advent of patient-centered medical home concepts in adult primary care (joint principles were published in 2007).24 Establishing team-based care in primary care practices has the potential to improve a variety of diagnostic processes through better communication and coordination within the primary care practice and with specialists; the evaluation of presenting symptoms such as rectal bleeding may particularly benefit from such concepts and practices.24,25,–26 Secondly, many studies also occurred prior to the widespread adoption of electronic health record (EHR) systems in Massachusetts (prevalence increased from approximately 18% in 2005 to at least 75% in 2011).27,28,–29 Greater EHR capacity in primary care facilitates the inclusion of clinical information that was previously not as easily captured by investigators, such as visit patterns and reasons (e.g., how frequently patients are seeing their primary care physicians vs. specialists), in the examination of factors that may impact the diagnostic evaluation of rectal bleeding but have not been included in prior studies. Lastly, prior studies were being conducted just as some clinical guidelines for rectal bleeding were becoming available (e.g., the tool developed by a task force of primary care physicians and gastroenterologists with support from the Controlled Risk Insurance Company [CRICO]).30,31
We were interested in whether the changes in clinical practice had improved the ability of physicians and other members of primary care teams to evaluate rectal bleeding, to document related activities such as colonoscopy orders/referrals, care coordination, and communication efforts, and to mitigate differences in care based on patient sex, race/ethnicity, or income.16,21,32
The aim of this study was to examine the degree to which primary care physicians document risk factors for colorectal cancer among patients with rectal bleeding and order colonoscopies when indicated, and the likelihood of physicians ordering and patients receiving recommended colonoscopies based on patients’ demographic characteristics, visit patterns, and clinical presentations.
METHODS
Study Design
We used explicit chart abstraction methods to conduct a cross-sectional study of 300 patients presenting to primary care with rectal bleeding between 2012 and 2016.
Study Setting
We studied patients receiving care at 15 primary care practices affiliated with Harvard Medical School. Between 2012 and 2016, these practices participated in two learning collaboratives. The first collaborative (2012–2014) was aimed at establishing or strengthening team-based care.25 The subsequent (2014–2016) learning collaborative focused on improving patient safety in the primary care setting, particularly by improving screening processes for breast and colorectal cancer and reducing preventable harm for patients with complex care needs. Both collaboratives were focused on system factors that could affect care for rectal bleeding processes, but not specifically on the diagnostic evaluation of rectal bleeding.25,33 All practices had EHRs prior to the start of the first learning collaborative in 2012, and all practices had established team-based care by 2014 as part of their participation in the collaborative. At the time, formal National Committee for Quality Assurance PCMH recognition was being pursued by nine practices, and the remainder had not started the process.
Data Sources and Study Population
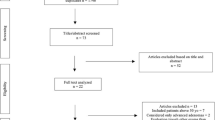
Our primary data sources were the administrative and clinical data present within each practice’s EHR (4 systems across 15 practices [e.g., Epic, eClinicalWorks]). We randomly sampled 20 patients from each practice across the 4 study years, for a total of 300 patients. In order to assess how care processes might differ by patient income/insurance, we over-sampled for Medicaid-insured patients and non-Medicaid patients in a 1:1 manner. We identified patients with rectal bleeding using International Classification of Diseases (ICD) versions 9 and 10 (Online Appendix Table 1). We included patients aged 40–80 years of age and excluded those who had a personal history of colorectal cancer, inflammatory bowel disease, or Lynch syndrome, and patients who left their primary care practice before the end of the abstraction period.
Chart Abstraction
Instrument Development
We based the chart abstraction instrument for this study on CRICO’s 2014 clinical guideline, “Prevention and Early Detection of Colorectal Cancer” (Online Appendix Figure 1), which is also in concordance with the American Society of Gastrointestinal Endoscopy’s guidelines for lower gastrointestinal bleeding.30 CRICO’s guideline recommends colonoscopies for most patients presenting with rectal bleeding, with the exception of patients aged 40–50 who have a negative family history (flexible sigmoidoscopy is allowed but colonoscopy is preferred for these patients), or patients over the age of 50 who have received a negative colonoscopy within the past 2 years (in these cases the physician may want to consider ordering a flexible sigmoidoscopy or repeat colonoscopy).31
The chart abstraction instrument captured patient information that began with initial presentation to primary care and continued until the patient received communication of the results of an indicated colonoscopy or 1 year had elapsed, whichever occurred first. In total, the abstraction instrument contained 10 domains and took a mean of 77 min (SD 68) to complete. Domains included the following: 1) visit patterns (e.g., total number of primary care or specialist visits or number of no-shows during the abstraction period), 2) patient demographic information (e.g., age, sex, race/ethnicity), 3) screening history (e.g., timing of prior colonoscopy, documentation of fecal occult blood testing if no prior colonoscopy), 4) signs and symptoms concerning for colorectal cancer, 5) problem list (containing both acute and chronic conditions) at initial presentation, 6) personal and family history of cancer or adenomas, 7) medications, 8) physical exam during the initial visit including perianal/rectal exam and stool occult blood test, 9) laboratory tests obtained (e.g., complete blood count), and 10) colonoscopy orders either placed as orders or via referrals made to gastroenterologists (procedure for initiating colonoscopy orders differed in these two ways across sites). The chart abstraction instrument also captured follow-up of planned care, including relevant information from subsequent primary care or specialist visits and procedures pertaining to the diagnostic evaluation of rectal bleeding.
The study team trained three bachelor’s level research assistants on a set of practice charts until they established a 93% agreement level with gold-standard abstractions that were established with the clinicians on our author team (SP, LP, ATC). The research assistants then double-abstracted a random subset of 10% of patients’ charts. To verify the reliability of the chart abstraction process throughout the data collection period, we used a weighted Cohen’s kappa, because response options in the chart abstraction tool could range from two to nine items. The resulting Cohen’s kappa was 0.95; the clinicians on our author team adjudicated disagreements.
Outcome Variables
Our main outcomes of interest were 1) the frequency at which risk factors for colorectal cancer and factors suggestive of alternative diagnoses for rectal bleeding were documented, 2) the frequency at which physicians ordered colonoscopies and patients received them, and 3) the odds of patients receiving a recommended colonoscopy. The procedure for ordering colonoscopies differed across sites. In approximately two-thirds of cases, physicians ordered colonoscopies directly, and in one-third of cases they referred patients to gastroenterology for colonoscopies; we considered both as “orders.”
Covariates
For our examination of factors that affected the odds of receiving a recommended colonoscopy, we included variables for patient characteristics—age, sex, race/ethnicity, insurance type, and number of concurrent chronic conditions at presentation (using the Agency for Healthcare Research and Quality Chronic Conditions Indicator [AHRQ CCI])—and time since most recent colonoscopy.34 We also included variables for visit patterns during the abstraction period (number of primary care and specialty visits unrelated to rectal bleeding and number of no-shows to primary care) and clinical presentation (documentation of risk factors for colorectal cancer and risk factors for alternative diagnoses at presentation).
Analyses
We used descriptive statistics to examine patient characteristics, visit patterns during the abstraction period, and clinical presentation. We used multivariable logistic regression to examine odds of physicians delivering a recommended colonoscopy. All tests were conducted with Stata 14 statistical software (StataCorp LP, College Station, TX).
The institutional review board at the Harvard T.H. Chan School of Public Health approved this study.
RESULTS
Our study population had a mean age of 56 (SD 10) years, 45% were female, 50% had Medicaid insurance, and 63% were non-white/Latino (i.e., Hispanic/Latino, African American, Asian/Pacific Islander, or other/unknown; Table 1). On average, patients had 2.6 (SD 2.0) concurrent chronic conditions documented in their problem lists at initial presentation. About half of patient charts had documentation of a prior colonoscopy: 31% of patients had one within the past 5 years, and 17% had one 5 or more years prior to presentation. Patients made on average 1.7 (SD 2.8) visits unrelated to rectal bleeding to primary care and 4.3 (SD 5.9) to subspecialists; they did not keep 0.4 (SD 1.0) appointment to primary care.
The frequency with which different risk factors for colorectal cancer and corresponding physical exam findings were documented in patient charts at the time of initial presentation was found to vary. The presence or absence of anemia was documented 9% of the time, personal or family history of adenomas 12–22%, unintentional weight loss 24%, and change in bowel habits 66% of the time (Table 2). The same was true for risk factors that could support alternative diagnoses for rectal bleeding: information about diverticulosis and hemorrhoids was captured in 13% and 47% of charts, respectively.
When risk factors and physical exam findings were documented, conditions were present as infrequently as 8% for unintentional weight loss and as often as 100% of the time in the case of diverticulosis. Rectal exams were documented at a frequency of 59%, and stool occult blood tests were documented in 35% of patients; the rectal exam was positive for hemorrhoids in 40% of documented cases, and stool occult blood tests were positive in 30% of documented cases.
CRICO’s clinical guideline recommended a colonoscopy for 89% (N = 268) of the 300 patients evaluated for rectal bleeding (Fig. 1). Of these 268 patients, physicians placed orders for colonoscopies for 74%. Physicians did not order colonoscopies during the abstraction period for 25% of patients. Four patients (1%) received colonoscopies because routine screening colonoscopies were scheduled prior to initial presentation. Of the orders that physicians placed, 85% occurred at initial presentation and 15% at a follow-up visit. Overall, 149 (56%) of the patients for whom a colonoscopy was recommended completed it within a year, and 96 (36%) of these colonoscopies were completed within 60 days. Most (87%) of the colonoscopies performed detected at least one abnormal finding, such as adenomas (30%), hemorrhoids (59%), and/or benign polyps (19%). One patient was diagnosed with colorectal cancer (Online Appendix Table 2).
Patient age and the timing of the most recent colonoscopy were significantly associated with colonoscopy orders being placed. Patients aged 50–64 years were more likely to have a colonoscopy order than those younger than 50 (OR = 2.2, 95% CI: 1.04, 4.8). Physicians were more likely to order a colonoscopy if the patient’s last colonoscopy was 5 or more years prior to presentation (OR = 4.04, 95% CI: 1.50,10.84) or if no prior colonoscopy was documented (OR = 2.64, 95% CI: 1.18, 5.91) compared to those whose most recent colonoscopy had occurred within the last 5 years (Table 3).
In contrast, the odds of patients receiving a recommended colonoscopy did not differ significantly according to the timing of a previous colonoscopy. However, the odds of patients receiving a recommended colonoscopy differed significantly with respect to patients’ visit patterns (Table 4). Patients who had more primary care visits during the abstraction period that were unrelated to rectal bleeding had significantly lower odds of receiving the test (OR = 0.78, 95% CI: 0.65, 0.94). We found no significant differences in patients’ odds of receiving a recommended colonoscopy based on patient age, racial/ethnic backgrounds, sex, insurance type, number of concurrent chronic conditions, time to most recent colonoscopy, or no-shows to primary care. Similarly, there were no significant differences in the odds of patients receiving a colonoscopy based on the assessment or presence of risk factors concerning for colorectal cancer or risk factors suggestive of alternative diagnoses for rectal bleeding.
DISCUSSION
Our study revealed that only 56% of patients presenting to primary care with rectal bleeding received a recommended colonoscopy within 1 year of presentation, with just 36% of colonoscopies occurring within 60 days of presentation. Further, among patients for whom colonoscopy orders or referrals were placed, only half were performed. The reasons for physician deviation from CRICO recommendations seems to involve several different types of factors: physicians documented risk factors for colorectal cancer less than half the time, and identification of risk factors did not modify the odds of colonoscopy completion. Together, these findings suggest that there remains a substantial need to improve the quality and consistency of diagnostic processes for patients presenting to primary care for rectal bleeding. Existing clinical recommendations offer physicians limited guidance in risk stratification to identify patients with rectal bleeding at highest risk of colorectal cancer, so physicians may be relying more on how much time has passed since a patient’s most recent colonoscopy. Additionally, the low completion rates for colonoscopies ordered suggests the need for systems to improve communication and/or care coordination efforts among primary care physicians, gastroenterologists, and patients.
Compared to a previous study, physicians documented rectal exams at a higher level; however, still 41% of patients were not examined.21 Physicians initiated colonoscopies for 74% of the patients for whom they were recommended by the CRICO guideline, but only 56% of patients received them during the study period (within 1 year of initial presentation). This rate is comparable to that in prior studies,15,21,22 despite the advent of the patient-centered medical home concept24,25 and more widespread adoption of electronic health records.26,27,–28
A recently published study in the UK and Fisher’s investigation of a VA system in the United States revealed delays in CRC diagnosis in patients with comorbid conditions.23,35 In our study, the number of comorbid chronic conditions was not associated with receipt of colonoscopy. However, we did observe that even after adjusting for the number of chronic conditions, patients with more primary care visits unrelated to rectal bleeding were less likely to complete colonoscopies that were ordered, suggesting that patients and physicians may have been attending to competing health priorities rather than pursuing evaluation of the rectal bleeding. Patients from non-white racial/ethnic backgrounds and with low-income/Medicaid insurance had the same likelihood of receiving recommended colonoscopies as those from white racial/ethnic backgrounds and commercial or Medicare insurance. Shields et al. performed an evaluation of rectal bleeding from 2006 to 2008 in the same primary care network, showing significant disparities in care.22 Our findings may represent a change in practice patterns or interventions aimed at detecting patients at risk of diagnostic delay in order to prevent such delays. Studies have demonstrated that alert systems such as electronic health record-based trigger algorithms and the use of patient navigator programs can help improve cancer screening rates and prevent prolonged diagnostic evaluation.36,37,38,39,40,41,–42 The similar odds of delivering a colonoscopy for low-income and non-white patients may be due in part to additional care coordination or patient navigation personnel that were present in all practices during the study. Previous research has shown that patient navigation can reduce disparities in colorectal cancer prevention for racial/ethnic minority populations.37,38,42,43,44,–45
Our study’s main limitation is that it relied on information documented in patient charts, which may not have represented all the care that was delivered. However, documented care is what quality improvement interventions rely on to understand how to improve clinical performance, and what malpractice claims rely upon in court cases, so documented care is a very important source of information about patient care. Additionally, in many ways, the electronic health record systems that we accessed to conduct this study enabled us to take account of data that were previously not as readily available, such as information about visit patterns that may help explain lower colonoscopy rates. A second important limitation of our study is the unknown generalizability to non-academic primary care practices or to other academic centers.
In summary, care for primary care patients presenting with rectal bleeding may be suboptimal. A stronger evidence base to guide clinical decision-making may be helpful for improving timely diagnosis of colorectal cancer, particularly when patients present with rectal bleeding. Given the complexity of the diagnostic evaluation of rectal bleeding, comprehensive assessments at initial presentation, improved care coordination across primary care and specialty settings, and the implementation of targeted interventions may help facilitate risk stratification and prevent missed or delayed diagnoses of colorectal cancer.
References
American Cancer Society. Cancer Facts & Figures. 2016. American Cancer Society. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2016.html. Published 2016. Accessed 2 November 2017.
Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review Br J Cancer. 2015;112:S92–107. doi:https://doi.org/10.1038/bjc.2015.48.
Thompson MR, Asiimwe A, Flashman K, Tsavellas G. Is earlier referral and investigation of bowel cancer patients presenting with rectal bleeding associated with better survival? Color Dis. 2011;13(11):1242–8. doi:https://doi.org/10.1111/j.1463-1318.2010.02438.x.
Talley NJ, Jones M. Self-reported rectal bleeding in a United States community: prevalence, risk factors, and health care seeking. Am J Gastroenterol. 1998;93(11):2179–83. doi:https://doi.org/10.1111/j.1572-0241.1998.00530.x.
Eslick GD, Kalantar JS, Talley NJ. Rectal bleeding: epidemiology, associated risk factors, and health care seeking behaviour: a population-based study. Color Dis. 2009;11(9):921–6. doi:https://doi.org/10.1111/j.1463-1318.2008.01721.x.
Crosland A, Jones R. Rectal bleeding: prevalence and consultation behaviour. BMJ. 1995;311(7003):486–8. doi:https://doi.org/10.1136/bmj.311.7003.486.
Zuckerman GR, Prakash C. Acute lower intestinal bleeding. Part II: etiology, therapy, and outcomes. Gastrointest Endosc. 1999;49(2):228–38. doi:https://doi.org/10.1016/S0016-5107(99)70491-8.
Astin M, Griffin T, Neal RD, Rose P, Hamilton W. The diagnostic value of symptoms for colorectal cancer in primary care: a systematic review. Br J Gen Pract. 2011;61(586):231–43. doi:https://doi.org/10.3399/bjgp11X572427.
Tørring ML, Murchie P, Hamilton W, et al. Evidence of advanced stage colorectal cancer with longer diagnostic intervals: a pooled analysis of seven primary care cohorts comprising 11 720 patients in five countries. Br J Cancer. 2017. doi:https://doi.org/10.1038/bjc.2017.236.
Bishop TF, Ryan AM, Ryan AK, Casalino LP. Paid malpractice claims for adverse events in inpatient and outpatient settings. JAMA. 2011;305(23):2427–31. doi:https://doi.org/10.1001/jama.2011.813.
Gandhi TK, Kachalia A, Thomas EJ, et al. Missed and delayed diagnoses in the ambulatory setting: a study of closed malpractice claims. Ann Intern Med. 2006;145(7):488–96. doi: https://doi.org/10.7326/0003-4819-145-7-200610030-00006.
Schiff GD, Puopolo AL, Huben-Kearney A, et al. Primary Care Closed Claims Experience of Massachusetts Malpractice Insurers. JAMA Intern Med. 2013;173(22):2063. doi:https://doi.org/10.1001/jamainternmed.2013.11070.
Ford AC, Veldhuyzen van Zanten SJO, Rodgers CC, Talley NJ, Vakil NB, Moayyedi P. Diagnostic utility of alarm features for colorectal cancer: systematic review and meta-analysis. Gut. 2008;57(11):1545–53. doi:https://doi.org/10.1136/gut.2008.159723.
Wahls TL, Peleg I. Patient- and system-related barriers for the earlier diagnosis of colorectal cancer. BMC Fam Pract. 2009;10(1):65. doi:https://doi.org/10.1186/1471-2296-10-65.
Weingart SN, Stoffel EM, Chung DC, et al. Delayed Workup of Rectal Bleeding in Adult Primary Care: Examining Process-of-Care Failures. Jt Comm J Qual Patient Saf. 2017;43(1):32–40. doi:https://doi.org/10.1016/j.jcjq.2016.10.001.
Singh H, Khan R, Giardina TD, et al. Postreferral colonoscopy delays in diagnosis of colorectal cancer: a mixed-methods analysis. Qual Manag Health Care. 2012;21(4):252–61. doi:https://doi.org/10.1097/QMH.0b013e31826d1f28.
Tomlinson C, Wong C, Au H-J, Schiller D. Factors associated with delays to medical assessment and diagnosis for patients with colorectal cancer. Can Fam Physician. 2012;58(9):e495–501. http://www.ncbi.nlm.nih.gov/pubmed/22972740. Accessed 2 November 2017.
Siminoff LA, Rogers HL, Thomson MD, Dumenci L, Harris-Haywood S. Doctor, what’s wrong with me? Factors that delay the diagnosis of colorectal cancer. Patient Educ Couns. 2011;84(3):352–8. doi:https://doi.org/10.1016/j.pec.2011.05.002.
Poon EG, Kachalia A, Puopolo AL, Gandhi TK, Studdert DM. Cognitive Errors and Logistical Breakdowns Contributing to Missed and Delayed Diagnoses of Breast and Colorectal Cancers: A Process Analysis of Closed Malpractice Claims. J Gen Intern Med. 2012;27(11):1416–23. doi:https://doi.org/10.1007/s11606-012-2107-4.
Allen AS, Orav EJ, Lee TH, Sequist TD. Clinician personality and the evaluation of higher-risk patient symptoms. J Patient Saf. 2011;7(3):122–6. doi:https://doi.org/10.1097/PTS.0b013e318223cb41.
Weingart SN, Stoffel EM, Chung DC, et al. Working up rectal bleeding in adult primary care practices. J Eval Clin Pract. 2016. doi:https://doi.org/10.1111/jep.12596.
Shields HM, Stoffel EM, Chung DC, et al. Disparities in evaluation of patients with rectal bleeding 40 years and older. Clin Gastroenterol Hepatol. 2014;12(4):669–75; quiz e33. doi:https://doi.org/10.1016/j.cgh.2013.07.008.
Fisher DA, Zullig LL, Grambow SC, et al. Determinants of medical system delay in the diagnosis of colorectal cancer within the Veteran Affairs Health System. Dig Dis Sci. 2010;55(5):1434–41. doi:https://doi.org/10.1007/s10620-010-1174-9.
American Academy of Family Physicians, American Academy of Pediatrics, American College of Physicians, American Osteopathic Association. Joint Principles of the Patient-Centered Medical Home. 2007. http://www.aafp.org/media-center/releases-statements/all/previous/20070305pressrelease0.html. Accessed 2 November 2017.
Bitton A, Ellner A, Pabo E, et al. Launching the Harvard Medical School Academic Innovations Collaborative: Transforming primary care practice and education. Acad Med. 2014;89(9):1239–44. doi:https://doi.org/10.1097/ACM.0000000000000410.
Chien AT, Kyle MA, Peters AS, et al. The degree to which practice configuration, size, and composition change while practices establish teams. J Ambul Care Manage forthcoming.
Friedberg MW, Safran DG, Coltin KL, Dresser M, Schneider EC. Readiness for the Patient-Centered Medical Home: structural capabilities of Massachusetts primary care practices. J Gen Intern Med. 2009;24(2):162–9. doi:https://doi.org/10.1007/s11606-008-0856-x.
Xierali IM, Hsiao C-J, Puffer JC, et al. The rise of electronic health record adoption among family physicians. Ann Fam Med. 2013;11(1):14–9. doi:https://doi.org/10.1370/afm.1461.
Simon SR, McCarthy ML, Kaushal R, et al. Electronic health records: which practices have them, and how are clinicians using them? J Eval Clin Pract. 2008;14(1):43–7. doi:https://doi.org/10.1111/j.1365-2753.2007.00787.x.
Pasha SF, Shergill A, Acosta RD, et al. The role of endoscopy in the patient with lower GI bleeding. Gastrointest Endosc. 2014;79(6):875–85. doi:https://doi.org/10.1016/j.gie.2013.10.039.
Controlled Risk Insurance Company. Prevention & Early Detection of Colorectal Cancer: A CRICO Decision Support Tool. https://www.rmf.harvard.edu/guidescolorectal. Published 2014. Accessed 2 November 2017.
Zapka J, Taplin SH, Anhang Price R, Cranos C, Yabroff R. Factors in Quality Care—The Case of Follow-Up to Abnormal Cancer Screening Tests—Problems in the Steps and Interfaces of Care. JNCI Monogr. 2010;2010(40):58–71. doi:https://doi.org/10.1093/jncimonographs/lgq009.
Schiff GD, Bearden T, Hunt LS, et al. Primary Care Collaboration to Improve Diagnosis and Screening for Colorectal Cancer. Jt Comm J Qual Patient Saf. 2017;24(0):211–7. doi:https://doi.org/10.1016/j.jcjq.2017.03.004.
Agency for Healthcare Research and Quality. Chronic Condition Indicator (CCI) for ICD-9-CM. http://www.hcup-us.ahrq.gov/toolssoftware/chronic/chronic.jsp. Accessed 2 November 2017.
Mounce LTA, Price S, Valderas JM, Hamilton W. Comorbid conditions delay diagnosis of colorectal cancer: a cohort study using electronic primary care records. Br J Cancer. 2017;116(12):1536–43. doi:https://doi.org/10.1038/bjc.2017.127.
Murphy DR, Laxmisan A, Reis BA, et al. Electronic health record-based triggers to detect potential delays in cancer diagnosis. BMJ Qual Saf. 2014;23(1):8–16. doi:https://doi.org/10.1136/bmjqs-2013-001874.
Berkowitz SA, Percac-Lima S, Ashburner JM, et al. Building Equity Improvement into Quality Improvement: Reducing Socioeconomic Disparities in Colorectal Cancer Screening as Part of Population Health Management. J Gen Intern Med. 2015;30(7):942–9. doi:https://doi.org/10.1007/s11606-015-3227-4.
Percac-Lima S, Ashburner JM, Zai AH, et al. Patient Navigation for Comprehensive Cancer Screening in High-Risk Patients Using a Population-Based Health Information Technology System. JAMA Intern Med. 2016;176(7):930. doi:https://doi.org/10.1001/jamainternmed.2016.0841.
Leffler DA, Neeman N, Rabb JM, et al. An Alerting System Improves Adherence to Follow-up Recommendations From Colonoscopy Examinations. Gastroenterology. 2011;140(4):1166–73.e3. doi:https://doi.org/10.1053/j.gastro.2011.01.003.
Meyer AND, Murphy DR, Singh H. Communicating Findings of Delayed Diagnostic Evaluation to Primary Care Providers. J Am Board Fam Med. 2016;29(4):469–73. doi:https://doi.org/10.3122/jabfm.2016.04.150363.
Lee J-H, Fulp W, Wells KJ, Meade CD, Calcano E, Roetzheim R. Effect of Patient Navigation on Time to Diagnostic Resolution among Patients with Colorectal Cancer-Related Abnormalities. J Cancer Educ. 2014;29(1):144–50. doi:https://doi.org/10.1007/s13187-013-0561-2.
Reuland DS, Brenner AT, Hoffman R, et al. Effect of Combined Patient Decision Aid and Patient Navigation vs Usual Care for Colorectal Cancer Screening in a Vulnerable Patient Population. JAMA Intern Med. 2017;173(18):1725–32. doi:https://doi.org/10.1001/jamainternmed.2017.1294.
Lasser KE, Murillo J, Lisboa S, et al. Colorectal Cancer Screening Among Ethnically Diverse, Low-Income Patients. Arch Intern Med. 2011;171(10):906–12. doi:https://doi.org/10.1001/archinternmed.2011.201.
Wu CA, Mulder AL, Zai AH, et al. A population management system for improving colorectal cancer screening in a primary care setting. J Eval Clin Pract. 2016;22(3):319–28. doi:https://doi.org/10.1111/jep.12427.
Percac-Lima S, López L, Ashburner JM, Green AR, Atlas SJ. The longitudinal impact of patient navigation on equity in colorectal cancer screening in a large primary care network. Cancer. 2014;120(13):2025–31. doi:https://doi.org/10.1002/cncr.28682.
Prior Presentations
This study was presented at the 40th Society of General Internal Medicine Annual Meeting in Washington, DC, April 2017.
Funding
Harvard Medical School Center for Primary Care; the Controlled Risk Insurance Company Risk Management Foundation of the Harvard Medical Institutions Incorporated. Sanja Percac-Lima was supported in part by the American Cancer Society Cancer Control Career Development Award for Primary Care Physicians, CCCDAA-14-012-01-CCCDA, and the Lazarex Cancer Foundation. While the funders supported the AIC and/or AIC CARES interventions, they were not involved in the design or conduct of the study, or the collection, management, analysis, or interpretation of the data, and had no role in shaping, approving, or deciding to submit the editorial content of this manuscript.
Author information
Authors and Affiliations
Contributions
The authors would like to thank Dr. Gordon Schiff for his comments on a pre-submission draft of this paper.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Rights and permissions
About this article
Cite this article
Percac-Lima, S., Pace, L.E., Nguyen, K.H. et al. Diagnostic Evaluation of Patients Presenting to Primary Care with Rectal Bleeding. J GEN INTERN MED 33, 415–422 (2018). https://doi.org/10.1007/s11606-017-4273-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-017-4273-x