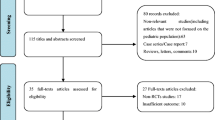
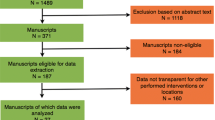
Abstract
Background
While several trials have compared laparoscopic to open surgery for colon cancer showing similar oncological results, oncological quality of laparoscopic versus open rectal resection is not well investigated.
Methods
A systematic literature search for randomized controlled trials was conducted in MEDLINE, the Cochrane Library, and Embase. Qualitative and quantitative meta-analyses of short-term (rate of complete resections, number of harvested lymph nodes, circumferential resection margin positivity) and long-term (recurrence, disease-free and overall survival) oncologic results were conducted.
Results
Fourteen randomized controlled trials were identified including 3528 patients. Patients in the open resection group had significantly more complete resections (OR 0.70; 95% CI 0.51–0.97; p = 0.03) and a higher number of resected lymph nodes (mean difference − 0.92; 95% CI − 1.08 to 0.75; p < 0.001). No differences were detected in the frequency of positive circumferential resection margins (OR 0.82; 95% CI 0.62–1.10; p = 0.18). Furthermore, no significant differences of long-term oncologic outcome parameters after 5 years including locoregional recurrence (OR 0.95; 95% CI 0.44–2.05; p = 0.89), disease-free survival (OR 1.16; 95% CI 0.84–1.58; p = 0.36), and overall survival (OR 1.04; 95% CI 0.76–1.41; p = 0.82) were found. Most trials exhibited a relevant risk of bias and several studies provided no information on the surgical expertise of the participating surgeons.
Conclusion
Differences in oncologic outcome between laparoscopic and open rectal surgery for rectal cancer were detected for the complete resection rate and the number of resected lymph nodes in favor of the open approach. No statistically significant differences were found in oncologic long-term outcome parameters.






Similar content being viewed by others
References
Siegel, R., C. Desantis, and A. Jemal, Colorectal cancer statistics, 2014. CA Cancer J Clin, 2014. 64(2): p. 104–17.
Edwards, B.K., et al., Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer, 2010. 116(3): p. 544–73.
Heald, R.J., E.M. Husband, and R.D. Ryall, The mesorectum in rectal cancer surgery—the clue to pelvic recurrence? Br J Surg, 1982. 69(10): p. 613–6.
Jacobs, M., J.C. Verdeja, and H.S. Goldstein, Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc, 1991. 1(3): p. 144–50.
van der Pas, M.H., et al., Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol, 2013. 14(3): p. 210–8.
Stead, M.L., et al., Assessing the relative costs of standard open surgery and laparoscopic surgery in colorectal cancer in a randomised controlled trial in the United Kingdom. Crit Rev Oncol Hematol, 2000. 33(2): p. 99–103.
Guillou, P.J., et al., Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet, 2005. 365(9472): p. 1718–26.
Theophilus, M., C. Platell, and K. Spilsbury, Long-term survival following laparoscopic and open colectomy for colon cancer: a meta-analysis of randomized controlled trials. Colorectal Dis, 2014. 16(3): p. O75–81.
Wang, C.L., G. Qu, and H.W. Xu, The short- and long-term outcomes of laparoscopic versus open surgery for colorectal cancer: a meta-analysis. Int J Colorectal Dis, 2014. 29(3): p. 309–20.
Di, B., et al., Laparoscopic versus open surgery for colon cancer: a meta-analysis of 5-year follow-up outcomes. Surg Oncol, 2013. 22(3): p. e39–43.
Kuhry, E., et al., Long-term outcome of laparoscopic surgery for colorectal cancer: a cochrane systematic review of randomised controlled trials. Cancer Treat Rev, 2008. 34(6): p. 498–504.
Vennix, S., et al., Laparoscopic versus open total mesorectal excision for rectal cancer. Cochrane Database Syst Rev, 2014(4): p. Cd005200.
Biondi, A., et al., Laparoscopic vs. open approach for colorectal cancer: evolution over time of minimal invasive surgery. BMC Surg, 2013. 13 Suppl 2: p. S12.
Panic, N., et al., Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS One, 2013. 8(12): p. e83138.
Higgins, J.P., et al., The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj, 2011. 343: p. d5928.
Bonjer, H.J., C.L. Deijen, and E. Haglind, A Randomized Trial of Laparoscopic versus Open Surgery for Rectal Cancer. N Engl J Med, 2015. 373(2): p. 194.
Jayne, D.G., et al., Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg, 2010. 97(11): p. 1638–45.
Jayne, D.G., et al., Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol, 2007. 25(21): p. 3061–8.
Green, B.L., et al., Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg, 2013. 100(1): p. 75–82.
Kang, S.B., et al., Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol, 2010. 11(7): p. 637–45.
Jeong, S.Y., et al., Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol, 2014. 15(7): p. 767–74.
Leung, K.L., et al., Laparoscopic resection of rectosigmoid carcinoma: prospective randomised trial. Lancet, 2004. 363(9416): p. 1187–92.
Ng, S.S., et al., Long-term morbidity and oncologic outcomes of laparoscopic-assisted anterior resection for upper rectal cancer: ten-year results of a prospective, randomized trial. Dis Colon Rectum, 2009. 52(4): p. 558–66.
Braga, M., et al., Laparoscopic resection in rectal cancer patients: outcome and cost-benefit analysis. Dis Colon Rectum, 2007. 50(4): p. 464–71.
Fleshman, J., et al., Effect of Laparoscopic-Assisted Resection vs Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: The ACOSOG Z6051 Randomized Clinical Trial. JAMA, 2015. 314(13): p. 1346–55.
Liu, F.L., et al., Hand-assisted laparoscopic surgery versus the open approach in curative resection of rectal cancer. J Int Med Res, 2010. 38(3): p. 916–22.
Lujan, J., et al., Randomized clinical trial comparing laparoscopic and open surgery in patients with rectal cancer. Br J Surg, 2009. 96(9): p. 982–9.
Ng, S.S., et al., Laparoscopic-assisted versus open total mesorectal excision with anal sphincter preservation for mid and low rectal cancer: a prospective, randomized trial. Surg Endosc, 2014. 28(1): p. 297–306.
Pechlivanides, G., et al., Lymph node clearance after total mesorectal excision for rectal cancer: laparoscopic versus open approach. Dig Dis, 2007. 25(1): p. 94–9.
Stevenson, A.R., et al., Effect of Laparoscopic-Assisted Resection vs Open Resection on Pathological Outcomes in Rectal Cancer: The ALaCaRT Randomized Clinical Trial. JAMA, 2015. 314(13): p. 1356–63.
Zhou, Z.G., et al., Laparoscopic versus open total mesorectal excision with anal sphincter preservation for low rectal cancer. Surg Endosc, 2004. 18(8): p. 1211–5.
Liang, X., et al., Effectiveness and safety of laparoscopic resection versus open surgery in patients with rectal cancer: a randomized, controlled trial from China. J Laparoendosc Adv Surg Tech A, 2011. 21(5): p. 381–5.
Kennedy, R.H., et al., Multicenter randomized controlled trial of conventional versus laparoscopic surgery for colorectal cancer within an enhanced recovery programme: EnROL. J Clin Oncol, 2014. 32(17): p. 1804–11.
Strobel, O. and M.W. Buchler, The problem of the poor control arm in surgical randomized controlled trials. Br J Surg, 2013. 100(2): p. 172–3.
Bege, T., et al., The learning curve for the laparoscopic approach to conservative mesorectal excision for rectal cancer: lessons drawn from a single institution’s experience. Ann Surg, 2010. 251(2): p. 249–53.
Kayano, H., et al., Evaluation of the learning curve in laparoscopic low anterior resection for rectal cancer. Surg Endosc, 2011. 25(9): p. 2972–9.
Son, G.M., et al., Multidimensional analysis of the learning curve for laparoscopic rectal cancer surgery. J Laparoendosc Adv Surg Tech A, 2010. 20(7): p. 609–17.
Leibold, T., et al., Prognostic implications of the distribution of lymph node metastases in rectal cancer after neoadjuvant chemoradiotherapy. J Clin Oncol, 2008. 26(13): p. 2106–11.
Kim, C.H., et al., Learning curve of laparoscopic low anterior resection in terms of local recurrence. J Surg Oncol, 2014. 110(8): p. 989–96.
Sauer, R., et al., Adjuvant vs. neoadjuvant radiochemotherapy for locally advanced rectal cancer: the German trial CAO/ARO/AIO-94. Colorectal Dis, 2003. 5(5): p. 406–15.
Funding
No funding was used to create this review. However, the resources and facilities of the University of Heidelberg were used in conducting this study.
Author information
Authors and Affiliations
Contributions
HN, PH, RS, BPM, ALM, and TS are responsible for the conception and design of the study. HN, PH, RS, ALM, and TS performed the acquisition and analysis of the data and drafted the manuscript. YK, MKD, JK, MS, BPM, AU, and MWB offered substantial contributions to the interpretation of the data and critically revised the manuscript. All authors gave their final approval of this version of the manuscript and are accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 67 kb)
Rights and permissions
About this article
Cite this article
Nienhüser, H., Heger, P., Schmitz, R. et al. Short- and Long-Term Oncological Outcome After Rectal Cancer Surgery: a Systematic Review and Meta-Analysis Comparing Open Versus Laparoscopic Rectal Cancer Surgery. J Gastrointest Surg 22, 1418–1433 (2018). https://doi.org/10.1007/s11605-018-3738-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-018-3738-5