Abstract
Purpose
To evaluate the utility of ring-type dedicated breast positron emission tomography (dbPET) for the detection of the residual tumor after neoadjuvant chemotherapy (NAC).
Materials and methods
This prospective study included 27 women with histologically proven breast cancer over a 37-month period. All patients underwent ring-type dbPET followed by whole-body PET-CT (WBPET) for preoperative tumor evaluation and re-staging after NAC. The maximum standardized uptake value (SUVmax) of the tumor lesion and the degree of confidence for the presence of the residual tumor were compared between pathological complete response (pCR) and non-pCR tumors. The sensitivity, specificity, and area under the receiver operating characteristic curve (AUC) for the detection of a non-pCR tumor were compared between dbPET and WBPET.
Results
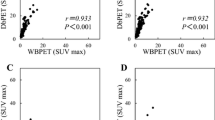
On dbPET, SUVmax was significantly higher in non-pCR than in pCR tumors (P = 0.030). The sensitivity for the detection of a non-pCR tumor was significantly higher with dbPET than with WBPET (84.2% vs 26.3%, P = 0.001). In the qualitative analysis, the sensitivity for the detection of a non-pCR tumor was also significantly higher with dbPET than with WBPET (57.9% vs 21.1%, P = 0.016).
Conclusion
The dbPET can provide more sensitive detection of residual tumor after NAC than can WBPET.


Similar content being viewed by others
References
Groheux D, Espie M, Giacchetti S, Hindie E. Performance of FDG PET/CT in the clinical management of breast cancer. Radiology. 2013;266(2):388–405.
Bos R, van Der Hoeven JJ, van Der Wall E, van Der Groep P, van Diest PJ, Comans EF, et al. Biologic correlates of (18)fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J Clin Oncol. 2002;20(2):379–87.
Brown RS, Wahl RL. Overexpression of glut-1 glucose transporter in human breast cancer. An immunohistochemical study. Cancer. 1993;72(10):2979–85.
Kumar R, Chauhan A, Zhuang H, Chandra P, Schnall M, Alavi A. Clinicopathologic factors associated with false negative FDG-PET in primary breast cancer. Breast Cancer Res Treat. 2006;98(3):267–74.
Avril N, Rose CA, Schelling M, Dose J, Kuhn W, Bense S, et al. Breast imaging with positron emission tomography and fluorine-18 fluorodeoxyglucose: use and limitations. J Clin Oncol. 2000;18(20):3495–502.
Thompson CJ, Murthy K, Weinberg IN, Mako F. Feasibility study for positron emission mammography. Med Phys. 1994;21(4):529–38.
Garcia Hernandez T, Vicedo Gonzalez A, Ferrer Rebolleda J, Sanchez Jurado R, Rosello Ferrando J, Brualla Gonzalez L, et al. Performance evaluation of a high resolution dedicated breast PET scanner. Med Phys. 2016;43(5):2261.
Nishimatsu K, Nakamoto Y, Miyake KK, Ishimori T, Kanao S, Toi M, et al. Higher breast cancer conspicuity on dbPET compared to WB-PET/CT. Eur J Radiol. 2017;90:138–45.
van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001;19(22):4224–37.
Choi M, Park YH, Ahn JS, Im YH, Nam SJ, Cho SY, et al. Assessment of pathologic response and long-term outcome in locally advanced breast cancers after neoadjuvant chemotherapy: comparison of pathologic classification systems. Breast Cancer Res Treat. 2016;160(3):475–89.
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet (Lond Engl). 2014;384(9938):164–72.
Mieog JS, van der Hage JA, van de Velde CJ. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst Rev. 2007;(2):Cd005002.
Yeh E, Slanetz P, Kopans DB, Rafferty E, Georgian-Smith D, Moy L, et al. Prospective comparison of mammography, sonography, and MRI in patients undergoing neoadjuvant chemotherapy for palpable breast cancer. AJR Am J Roentgenol. 2005;184(3):868–77.
Chen JH, Bahri S, Mehta RS, Kuzucan A, Yu HJ, Carpenter PM, et al. Breast cancer: evaluation of response to neoadjuvant chemotherapy with 3.0-T MR imaging. Radiology. 2011;261(3):735–43.
Kurosumi M, Akashi-Tanaka S, Akiyama F, Komoike Y, Mukai H, Nakamura S, et al. Histopathological criteria for assessment of therapeutic response in breast cancer (2007 version). Breast Cancer (Tokyo Jpn). 2008;15(1):5–7.
Dose-Schwarz J, Tiling R, Avril-Sassen S, Mahner S, Lebeau A, Weber C, et al. Assessment of residual tumour by FDG-PET: conventional imaging and clinical examination following primary chemotherapy of large and locally advanced breast cancer. Br J Cancer. 2010;102(1):35–41.
Eo JS, Chun IK, Paeng JC, Kang KW, Lee SM, Han W, et al. Imaging sensitivity of dedicated positron emission mammography in relation to tumor size. Breast (Edinb Scotl). 2012;21(1):66–71.
Kalinyak JE, Berg WA, Schilling K, Madsen KS, Narayanan D, Tartar M. Breast cancer detection using high-resolution breast PET compared to whole-body PET or PET/CT. Eur J Nucl Med Mol Imaging. 2014;41(2):260–75.
Yamamoto Y, Ozawa Y, Kubouchi K, Nakamura S, Nakajima Y, Inoue T. Comparative analysis of imaging sensitivity of positron emission mammography and whole-body PET in relation to tumor size. Clin Nucl Med. 2015;40(1):21–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
About this article
Cite this article
Koyasu, H., Goshima, S., Noda, Y. et al. The feasibility of dedicated breast PET for the assessment of residual tumor after neoadjuvant chemotherapy. Jpn J Radiol 37, 81–87 (2019). https://doi.org/10.1007/s11604-018-0785-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-018-0785-5